Latest addition to growing portfolio of breast and skeletal health innovations to be featured at ECR 2019
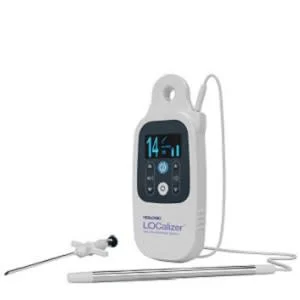
Hologic announced today the granting of a CE Mark to the LOCalizer™ wireless radio frequency identification (RFID) breast lesion localization system. This system is designed for precise and easy marking and targeting of lesions for breast-conserving surgery guidance.
The system is one of many in Hologic’s expanding suite of breast and skeletal health products, including screening, interventional, ultrasound and surgical solutions, that will be available for demonstration in Booths X2 - 211 and X5 - 521 at the annual European Congress of Radiology (ECR) meeting in Vienna, Austria from Feb. 27 to March 3.
The LOCalizer tag is designed to replace the traditional wire-guided localization method, helping to provide increased comfort and convenience for patients and their healthcare teams. The tag can be implanted up to 30 days prior to a breast-conserving surgery, providing increased flexibility for patients and providers. This improved workflow is designed to help reduce scheduling and logistical hurdles for care teams and aims to deliver added convenience for an enhanced patient experience. Following placement, the miniature implantable tag can be detected by a portable, handheld reader that indicates the location and distance in millimeters to the lesion, enabling the surgeon to pinpoint the correct area of breast tissue for removal.
“We look forward to showcasing the new LOCalizer system at ECR as we continue to broaden our offerings to make a positive impact on breast health at every step of the patient journey – from screening to pathology,” said Jan Verstreken, Regional President EMEA and Canada, Hologic. “This thoughtful expansion is rooted in our commitment to developing new and innovative solutions clinically proven to improve cancer detection, patient satisfaction and facility workflow, while reducing costs associated with unnecessary callbacks.”
The market leader behind the 3D Mammography™ exam, Hologic has expanded significantly in recent years through insight-driven innovation and strategic acquisitions to address the entire clinical continuum of breast health. Along with the LOCalizer system, the Company’s new products include the SmartCurve™ breast stabilization system, Clarity HD high-resolution 3D™ imaging technology, the Viera™ portable breast ultrasound system, and the Brevera® breast biopsy system with CorLumina® imaging technology, which features real-time imaging and sample verification. Two recent acquisitions of Faxitron® Bioptics, a leader in digital specimen radiography, and Focal Therapeutics, manufacturer of the BioZorb® marker, have enabled Hologic to play a larger role in breast-conserving surgery and strengthened its offerings to radiologists, pathologists and breast surgeons.
Visitors to the Hologic booth at ECR can experience the A.I. Future Suite, highlighting Hologic’s long-standing commitment to incorporating artificial intelligence and machine learning into its groundbreaking technologies. Attendees will have a chance to experience current and future applications of A.I. in breast imaging, and learn more about how Hologic plans to continue to deliver significant value as a leader in the space. In addition, the Company’s entire suite of breast and skeletal health products, such as the 3Dimensions™ Mammography System and Fluoroscan® Insight™ FD Mini C-Arm, will be on display and available for demonstration.
Throughout ECR, Hologic will host a variety of workshops and a symposium, Advances in Breast Imaging: Clinical use of CEDM across Europe. Workshop topics will offer hands-on experience and expert insight into topics including: clinical workflow using tomosynthesis guidance and real-time breast biopsy imaging; a new technique for breast lesion localization; wireless ultrasound guided biopsies; contrast-enhanced mammography in clinical practice; optimization of tomosynthesis reading time; and breast density case reviews. For more information, please visit ecr.Hologic.com or visit Hologic’s Booths X2-211 and X5-521.
The LOCalizer system is manufactured by Health Beacons, Inc. and is exclusively distributed by Hologic.
Click here to find out more about Hologic.
Latest Articles
Hologic, ECR 2019, 3D Mammography™ exam, LOCalizer Wireless Breast Lesion Localization System, LOCalizer™, Faxitron® Bioptics, Focal Therapeutics
Hologic announced today the granting of a CE Mark to the LOCalizer™ wireless radio frequency identification (RFID) breast lesion localization system. This system is designed for precise and easy marking and targeting of lesions for breast-conserving surge