Good news for men with prostate problems including prostate cancer and benign prostate enlargement.
A new minimally invasive method, the MRI-guided transurethral ultrasound ablation (TULSA), has been shown to be effective in treating prostate cancer with minimal side effects, according to a new study presented at the annual meeting of the Radiological Society of North America (RSNA).
You might also like: PSMA-based PET Radiotracers Transforming Care in Prostate Cancer
Researchers said that
clinically significant cancer was eliminated in 80% of the study participants,
adding that the incision-free technique could also be used to treat benign
enlargement of the prostate gland.
Treating disease in
the small gland that surrounds the urethra just outside the bladder is
challenging. Surgery and radiation are not always effective and can result in
incontinence, impotence and bowel dysfunction.
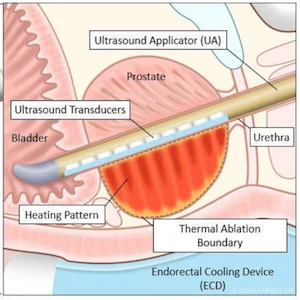
TULSA works by
delivering precise doses of sound waves to diseased prostate tissue, ensuring
the healthy nerve tissue around the prostate is not damaged. TULSA relies on a
rod-shaped device that is inserted into the urethra. The device has 10
ultrasound-generating elements enough to cover the entire prostate gland. One
or more of the elements are used to send out sound waves that heat and destroy
the target prostate tissue.
In the new multicentre
study, researchers reported on the 12-month outcomes from the TULSA-PRO®
ablation clinical trial (TACT). The trial enrolled 115 men, median age 65, with
localised low or intermediate risk, gland-confined prostate cancer. Clinicians
delivered TULSA treatment to the entire gland. Treatment time averaged 51
minutes. The entire procedure took place in an MRI scanner, allowing doctors to
monitor treatment and assess the degree and location of heating.
Key findings of the
clinical trial include:
- Prostate volume decreased on average from 39 cubic centimetres pre-treatment to 3.8 cubic cm a year after treatment;
- Clinically significant cancer was eliminated in 80% of the study patients, with 65% (72/111) showing no evidence of any cancer at biopsy after one year;
- Blood levels of prostate-specific antigen (PSA), a marker of prostate cancer, fell by a median of 95%; and
- There were low rates of severe toxicity and no bowel complications.
With the good results
from this study, the researchers were quick to cite the advantages of using
TULSA, which is already approved for clinical use in Europe. TULSA has just
received FDA 510(k) clearance for prostate tissue ablation in the United
States.
Study co-author Steven S. Raman, MD, professor of radiology and urology, and director of Prostate MR Imaging and Interventions and Prostate MR Imaging Research at the University of California at Los Angeles (UCLA), points out "two very unique things" about the new MRI-guided technique.
"First, you can
control with much more finesse where you're going to treat, preserving
continence and sexual function," he said. "Second, you can do this
for both diffuse and localised prostate cancer and benign diseases, including
benign hyperplasia."
TULSA also has the
benefit of allowing further treatment if needed. When it fails, then the
procedure can be repeated, and more aggressive invasive approaches like surgery
and radiation therapy can still be used, Dr Raman explained. Alternatively,
TULSA may enable noninvasive treatment for localised radiation failure.
Source: Radiological Society of North America
Image credit: Radiological Society of North America