Growing number of peer-reviewed clinical publications document significant advantages of Hologic® tomosynthesis; Now, Hologic has further advanced tomosynthesis with faster and lower-dose options including the C-View™ software option for 2D imaging and the Affirm™ tomosynthesis biopsy procedure.
Tomosynthesis is going from strength to strength. Several recently published clinical studies demonstrate that screening with Hologic tomosynthesis in combination with conventional 2D imaging results in higher detection rates (i-iii)
and lower false-positive rates.(iii)
In addition, Hologic has advanced tomosynthesis technology further with faster and lower dose options including C-View™ software for generating 2D images and the Affirm™ tomosynthesis biopsy option. These new procedures are being performed in increasing numbers across Europe, the U.S., and countries around the world.
European Data Shows Increased Detection, Decreased Recalls
Results of the first large-scale, prospective clinical study on breast tomosynthesis were published in April 2013 in the scientific journal Radiology. The study, “Comparison of Digital Mammography Alone and Digital Mammography plus Tomosynthesis in a Population-based Screening Program” was led by Per Skaane, M.D., Ph.D. from Oslo University Hospital Ullevaal. Well-respected in his field, Skaane and his research team conducted the previous Oslo I and Oslo II trials comparing digital mammography to screen-film for the detection of breast cancer.
The tomosynthesis study was based on 12,631 screening examinations in a large hospital in Norway. The researchers, using Hologic's breast tomosynthesis in combination with a 2D mammogram, found a significant increase in cancer detection rates, particularly for invasive cancers, and a simultaneous decrease in false-positive rates compared with 2D mammography alone. Significant findings include:
- A 40% increase in the detection of invasive breast cancers
- A 27% increase in the detection of all cancers (invasive and in situ cancers combined)
- A 15% decrease in false-positive rates
Another European study, "Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study," came to a similar conclusion. The study, led by a team of Italian and Australian researchers represented by Associate Professor Nehmat Houssami, at the University of Sydney's School of Public Health in Australia, was published in June 2013 in Lancet Oncology.
The study investigated whether integrated 2D mammography with tomosynthesis was more effective at detecting cancers and reducing false positives than 2D screening alone. Again, the use of Hologic's breast tomosynthesis technology significantly increased cancer detection rates and simultaneously reduced false positives. Significant findings include:
- Breast tomosynthesis identified 8.1 cancers per 1,000 screens, compared with 2D mammography alone at 5.3 per 1,000 screens, an increase in the number of cancers detected by over 50%.
- Breast tomosynthesis resulted in a simultaneous 17% recall rate reduction.

The Hologic Affirm™ breast biopsy guidance system, shown with the Eviva breast biopsy device on the Selenia® Dimensions® breast tomosynthesis system, may be used to perform tomosynthesis
biopsies.
U.S. Study Results Consistent with European Findings
These positive results can also be seen in the U.S., with the first large scale breast cancer screening trial reporting findings consistent with and supplementary to the Oslo Tomosynthesis Screening Trial and the Screening Tomosynthesis or Mammography (STORM) trial in Italy.
The study, "Implementation of Breast Tomosynthesis in a Routine Screening Practice: An Observational Study", compared breast cancer screening with Hologic’s breast tomosynthesis to conventional 2D mammography alone and showed a significant reduction in recall rates and a sizeable increase in cancer detection, particularly invasive cancer, across all breast tissue densities.
Published in the June issue of the American Journal of Roentgenology (AJR), the study was led by Stephen L. Rose, M.D., President and Founder of Rose Imaging, Medical Director of TOPS Comprehensive Breast Center, and Breast Radiologist affiliated with Memorial Hermann Health System in Houston. It evaluated recall, cancer detection and invasive cancer detection rates in a community-based breast imaging practice.
The Rose study found that the use of Hologic's breast tomosynthesis resulted in:
- A significant 38% drop in recall rates – from 8.7% to 5.5% (p < 0.001)
- A 35% increase in cancer detection rates - from 4.0 to 5.4 per 1,000 screenings (p = 0.18)
- A 53% increase in invasive cancer detection rates - from 2.8 to 4.3 per 1,000 screening examinations (p = 0.07)
The study population included 13,856 women who had received conventional 2D mammography screening exams and 9,499 women who received a Hologic tomosynthesis screening exam.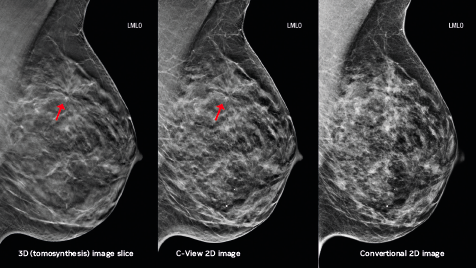

Hologic’s C-View software option provides a lower-dose solution by generating a 2D image from the tomosynthesis dataset, eliminating the need for a separate 2D exposure.
Ground-breaking Advances in Tomosynthesis
A lower-dose tomosynthesis option from Hologic, the C-View software option, has been commercially available in Europe and many countries in Latin America and Asia since 2011 and became available in the US in May 2013. Hologic's C-View software produces 2D images from 3D datasets. These generated 2D images may now be used in place of the conventional 2D exposure required by the US Food and Drug Administration (FDA) as part of a Hologic 3D mammography screening exam. The combination of Hologic's 3D and C-View 2D images results in a faster combined exam scan time (about 4 seconds) resulting in less time under compression for greater patient comfort and reduced risk of motion. While Hologic 3D mammography is well under the MQSA and EUREF limits and deemed safe, the 3D and C-View exam offers lower patient radiation dose compared to combo mode. In fact, 3D mammography procedures with C-View offers a total patient dose similar to a 2D only exam while providing superior clinical performance for all breast types.
Another important advance in breast tomosynthesis is the ability to perform a breast biopsy using tomosynthesis guidance. The Hologic Affirm™ breast biopsy guidance system, combined with a Selenia® Dimensions® breast tomosynthesis system and the Eviva® breast biopsy device are being used in increasing numbers in Europe and the U.S. for this purpose. The Affirm tomosynthesis biopsy procedure allows the localisation and accurate targeting of regions of interest and is especially important for targeting lesions not found in 2D images or in other modalities. This new biopsy technique has numerous advantages over traditional stereotactic biopsy procedures, including faster lesion targeting, lower patient dose and reduced patient procedure time.
For more information on this exciting technology, please contact us at [email protected]

Hologic, Affirm, C-View, Dimensions, Eviva and Selenia are trademarks and/or registered trademarks of Hologic, Inc., and/or its subsidiaries in the United States and/or other countries.
References:
i Skaane P, Bandos A, Gullien R, et al. “Comparison of Digital Mammography Alone and Digital Mammography Plus Tomosynthesis in a Population-based Screening Program.” Radiology. 2013 Apr; 267(1):47-56.
ii Ciatto S, Houssami N, Bernardi D, et. Al. “Integration of 3D Digital Mammography with Tomosynthesis for Population Breast-Cancer Screening (STORM): A Prospective Comparison Study” The Lancet Oncology, Epub 2013
Apr 25.
iii Rose S, Tidwell A, Bujnock L, et al. “Implementation of Breast Tomosynthesis in a Routine Screening Practice: An Observational Study.” American Journal of Roentengenology. 2013 Jun; 200(6): 1401-1408. Epub 2013 May 22.
iv FDA PMA submission P080003/S001
Latest Articles
Breast, Tomosynthesis, Hologic
Growing number of peer-reviewed clinical publications document significant advantages of Hologic® tomosynthesis; Now, Hologic has further advanced tomosyn...