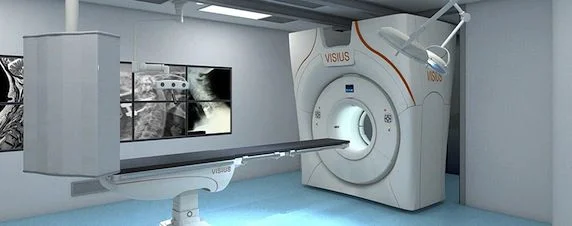
IMRIS Inc. has announced US Food and Drug Administration (FDA) 510K clearance to market VISIUS® iCT, the first and only ceiling-mounted intraoperative computed tomography (iCT) on the market.
“This revolutionary IMRIS solution will be an essential part of the hybrid operating room of the future, especially for the spinal and neurosurgical market,” said David Graves, IMRIS CEO. “VISIUS iCT provides surgeons with on-demand CT imaging to enhance decision making, and both guide and confirm implant placement. VISIUS iCT establishes a level of intraoperative imaging previously unavailable to surgeons and patients.”
VISIUS iCT is a state-of-the-art surgical theatre that provides personalised dose management together with diagnostic quality imaging during the surgical procedure to assist surgeons in critical decision making. The 64-slice scanner effortlessly moves into and out of the operating room during surgery using ceiling-mounted rails to ease workflow. This enables multiple room configurations to meet both clinical requirements and increase utilisation without compromising image quality or exam speed.
Patient transport and the need for floor-mounted rails is eliminated, which opens up valuable OR space and allows unimpeded movement of surgical equipment and simplified infection control. The system also offers the longest scanner travel range on the market today.
VISIUS iCT features a suite of software applications such as 3D volume rendering to aid in surgical planning and dose reduction which considers each patient’s unique characteristics and needs to maximise image quality and minimise dose. The system software allows healthcare practitioners to visualise dosage prior to scan and adjust settings based on the specific clinical need with detailed dosage reports produced after each scan.
IMRIS also announced on 5 August that it has received obtained regulatory CE mark for VISIUS® iCT, allowing for sales and marketing in the European Union. Jay D. Miller, IMRIS CEO and President, said, "European Union spine surgeons now have the opportunity to use this unique solution in confirming implant placements, fusion and other complex procedures. As procedures become more minimally invasive, the need for better visualization with advanced imaging increases. Both U.S. and European spine surgeons will be especially interested in the outstanding dose management capabilities and state-of-the-art image quality available with VISIUS iCT."
Latest Articles
CT
IMRIS Inc. has announced US Food and Drug Administration (FDA) 510K clearance to market VISIUS® iCT, the first and only ceiling-mounted intraoperative com...