Positron emission tomography (PET) is widely used in oncology and other areas of clinical practice for diagnosis, staging, treatment monitoring and follow-up. High-quality images are essential for detecting small lesions and guiding decisions, but image clarity is strongly linked to scan duration. While longer acquisitions improve image quality, they also increase the risk of motion artefacts and patient discomfort. These challenges are particularly evident in children or individuals who cannot remain still for extended periods, leading to potential misdiagnoses or missed lesions.
Ultrafast total-body PET imaging, enabled by advanced scanners such as the uEXPLORER, offers a potential solution by drastically reducing acquisition times. However, shorter scans typically produce noisier images, which limit diagnostic value. To address this, researchers developed a transformer-based deep learning framework designed to synthesise diagnostic-quality PET images from ultrafast scans across multiple tracers. Their analysis of [¹⁸F]FDG, [¹⁸F]FAPI and [⁶⁸Ga]FAPI datasets demonstrates that deep learning can improve both image quality and lesion detectability while reducing scan time, offering a feasible pathway to integrate ultrafast imaging into routine clinical workflows.
Deep Learning Models for Image Enhancement
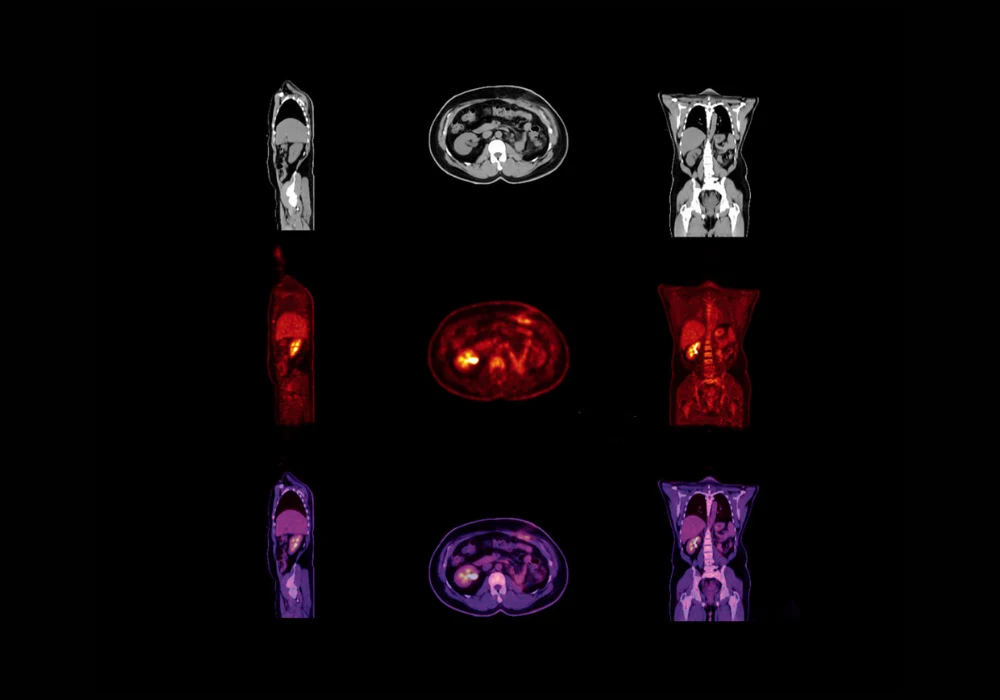
The research involved retrospective analysis of 155 patients who underwent total-body PET/CT imaging with [¹⁸F]FDG, [¹⁸F]FAPI and [⁶⁸Ga]FAPI tracers. Standard 300-second scans were acquired for each patient and served as the reference for comparison. Ultrafast images with acquisition times of 3, 6, 15, 30 and 40 seconds were generated by truncating list-mode data. Two models based on the SwinUNETR-V2 architecture were tested: Model 1, which used ultrafast PET input alone and Model 2, which incorporated both ultrafast PET and low-dose CT inputs.
Must Read: Shared Total-Body PET for Clinical and Research Use
Both models were trained and evaluated using five-fold cross-validation. The architecture was designed to preserve tumour and tissue structures while reducing noise. Visual comparisons showed that images produced by the models more closely resembled standard scans than the original ultrafast acquisitions. Importantly, Model 1 was particularly effective for the shortest scans, which were the noisiest and most difficult to interpret. Model 2 performed better for 30- and 40-second scans, where CT priors helped refine image quality. These differences suggest that each model has distinct advantages depending on the acquisition time and level of noise.
Quantitative Evaluation and Clinical Assessment
Objective image quality metrics supported the visual findings. Both models significantly improved peak signal-to-noise ratio, structural similarity index and correlation compared with unprocessed ultrafast scans. The greatest improvements were observed in 3-, 6- and 15-second acquisitions, which without enhancement were considered non-diagnostic. For 30- and 40-second scans, enhancements were more modest, as these acquisitions already approximated the quality of 300-second reference images. In some comparisons, the differences between the enhanced 30- or 40-second scans and full-length scans were not statistically significant, indicating that longer ultrafast acquisitions may be nearly sufficient on their own.
Clinical evaluation involved two nuclear medicine physicians who reviewed anonymised images across all tracers and scan times. They assessed image quality using a five-point grading scale and recorded lesion detectability. Both readers reported higher quality scores for the enhanced images than for the raw ultrafast scans, with improvements of at least 0.4 points across all acquisition times. Even ultrafast images as short as 3 seconds were upgraded from non-diagnostic to acceptable quality when processed through the models. Lesion detectability was also significantly improved. While ultrafast scans alone led to missed lesions, the enhanced images achieved detection rates comparable to standard scans. For acquisitions of 15 seconds or longer, both models consistently detected at least 69 lesions across readers, aligning closely with the reference images.
Impact on Clinical Practice and Patient Care
The ability to generate diagnostic PET images from ultrafast scans carries meaningful clinical implications. Breath-hold imaging becomes feasible, with acquisitions completed within 3–15 seconds, reducing respiratory motion artefacts and improving lesion clarity. This approach can be integrated into clinical workflows with minimal changes, using standard verbal instructions as in CT or MRI. For patients with conditions that limit their ability to remain still, such as Alzheimer’s disease, chronic pain or paediatric cases, tailored ultrafast protocols can provide usable images within their tolerance limits.
Shorter scan durations also mitigate the discomfort associated with the long axial field-of-view of total-body PET scanners, reducing claustrophobia and improving compliance. Operationally, reduced acquisition times increase scanner throughput, allowing more patients to be imaged within the same timeframe. Importantly, the models are efficient, capable of running parallel inference tasks on standard GPU hardware, which supports practical deployment in clinical settings.
While the findings are promising, limitations include the single-centre nature of the dataset and the relatively small cohort size. Broader validation across multiple institutions and larger patient populations will be essential to confirm generalisability. Further advances in network design may also enhance denoising performance, particularly for very short acquisitions.
Transformer-based deep learning models show strong potential in enabling ultrafast multi-tracer total-body PET imaging. Both models substantially improved image quality and lesion detectability, particularly for very short acquisitions that would otherwise be non-diagnostic. By reducing scan times without compromising diagnostic reliability, this approach can enhance patient comfort, minimise artefacts and optimise clinical workflows. The feasibility of integrating ultrafast PET imaging into standard practice is supported by the efficiency and adaptability of the models, which require minimal changes to existing protocols. These findings highlight a practical pathway for advancing PET imaging towards shorter, more patient-friendly examinations while maintaining high diagnostic standards.
Source: Academic Radiology
Image Credit: iStock