Detecting the return of prostate cancer after initial treatment remains a major challenge, particularly for patients with low prostate-specific antigen (PSA) levels. Conventional imaging methods often fail to identify disease recurrence at this early stage, limiting treatment options. Prostate-specific membrane antigen (PSMA) targeted PET imaging has emerged as a more precise approach, and recent phase 3 data from the GuidePath trial highlight the performance of [18F]CTT1057, a fluorine-18-labelled PSMA radiotracer, in this context. The findings provide evidence for its diagnostic accuracy in patients experiencing biochemical recurrence, predominantly at their first relapse, and suggest it may expand access to effective PET imaging in clinical practice.
Clinical Context and Study Design
Biochemical recurrence, indicated by a rising PSA level after radical prostatectomy or radiotherapy, affects around 40% of patients within ten years of treatment. Although PSA is a useful marker of relapse, it cannot localise disease accurately. Conventional imaging methods, such as bone scintigraphy or CT, are often insensitive at low PSA ranges, while multiparametric MRI has limited reach beyond the prostate bed. PSMA-targeted PET tracers, such as [68Ga]Ga-PSMA-11 and fluorine-18-labelled agents, have demonstrated higher efficacy, but clinical data in patients at their first recurrence with very low PSA levels have been scarce.
Must Read: Advancing Prostate Cancer Imaging: The Role of [68Ga]Ga-PSMA-R2
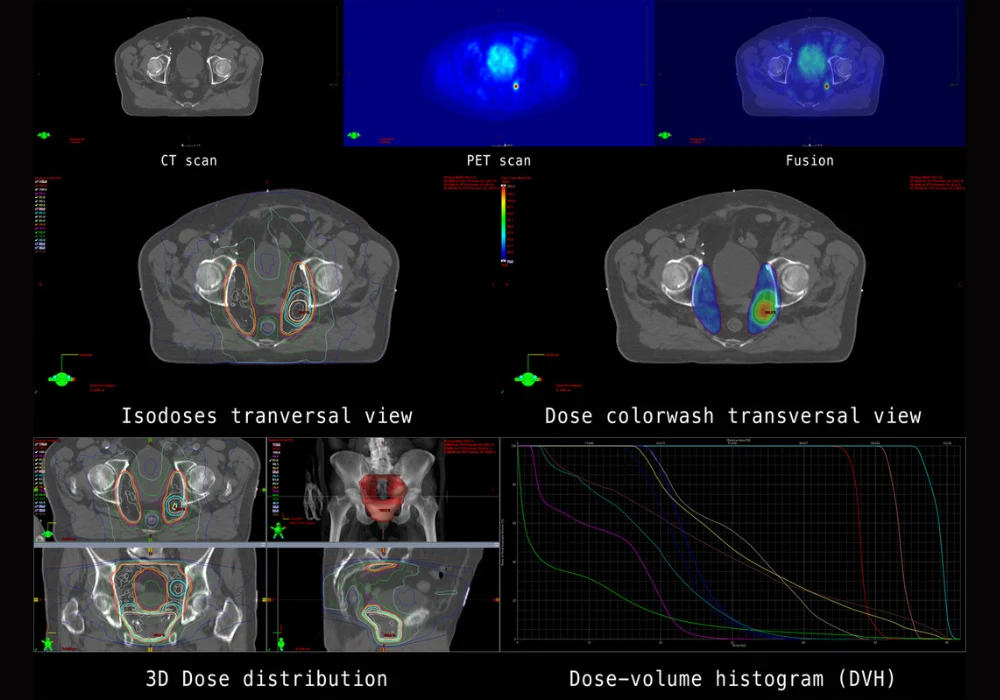
GuidePath, a prospective, multicentre phase 3 trial conducted across Europe and the United States, was designed to address this gap. Eligible patients had a PSA of 0.2 ng/mL or greater after surgery or an increase of at least 2 ng/mL above nadir following radiotherapy. Participants underwent PET/CT scans with both [18F]CTT1057 and [68Ga]Ga-PSMA-11, with imaging reviewed by three independent readers blinded to clinical data. Diagnostic performance was measured using a composite truth standard prioritising histopathology, dual-modality imaging and treatment response.
Diagnostic Accuracy and Performance
Of 202 patients screened, 161 were evaluable for efficacy, with most experiencing their first biochemical recurrence and having undergone radical prostatectomy as initial therapy. Median baseline PSA was 0.4 ng/mL, reflecting a clinically relevant low-level cohort. Both coprimary endpoints were met: the region-level correct localisation rate for [18F]CTT1057 ranged from 65.2% to 75.0%, while the patient-level positive predictive value ranged from 64.6% to 76.5%. These values surpassed predefined thresholds, confirming diagnostic utility in this patient population.
Performance was consistent across subgroups, although higher baseline PSA levels correlated with numerically improved results. Importantly, intended management plans changed in more than one third of patients following [18F]CTT1057 PET/CT imaging, underscoring its clinical impact. Interreader agreement was substantial, with all three readers concurring in 76% of scans, and intrareader reproducibility ranged from 61.2% to 100%.
Safety data indicated [18F]CTT1057 was well tolerated. Adverse events were infrequent, mostly mild and unrelated to the radiotracer. No grade 3 or higher events were reported, and no treatment-related deaths occurred.
Positioning Among Existing PET Tracers
GuidePath findings are notable when compared with previous phase 3 trials of fluorine-18-labelled PSMA tracers, including CONDOR for [18F]DCFPyL and SPOTLIGHT for [18F]rhPSMA-7.3. Unlike those studies, which enrolled patients with higher baseline PSA levels and prior salvage therapies, GuidePath focused on a population largely at first recurrence with lower PSA values. This design makes the results highly applicable to everyday clinical practice, where early detection is critical.
Region-level performance for [18F]CTT1057 compared favourably with SPOTLIGHT and was comparable to CONDOR, despite the lower PSA thresholds in the GuidePath cohort. The use of [68Ga]Ga-PSMA-11 PET as part of the reference standard further strengthened the reliability of results, overcoming limitations of conventional imaging comparators used in earlier trials. The tracer also demonstrated high accuracy in detecting skeletal lesions, potentially reducing issues of non-specific bone uptake that can affect other fluorinated PSMA agents.
GuidePath demonstrated that [18F]CTT1057 provides accurate detection of PSMA-positive lesions in patients with prostate cancer at their first biochemical recurrence, even at low PSA levels. Its diagnostic performance influenced treatment decisions in a substantial proportion of cases and confirmed favourable safety. By capturing a clinically common yet underrepresented patient group, these results support [18F]CTT1057 as a valuable addition to available PSMA PET radiotracers, with potential to broaden access to reliable imaging for recurrence management in prostate cancer.
Source: The Journal of Nuclear Medicine
Image Credit: iStock