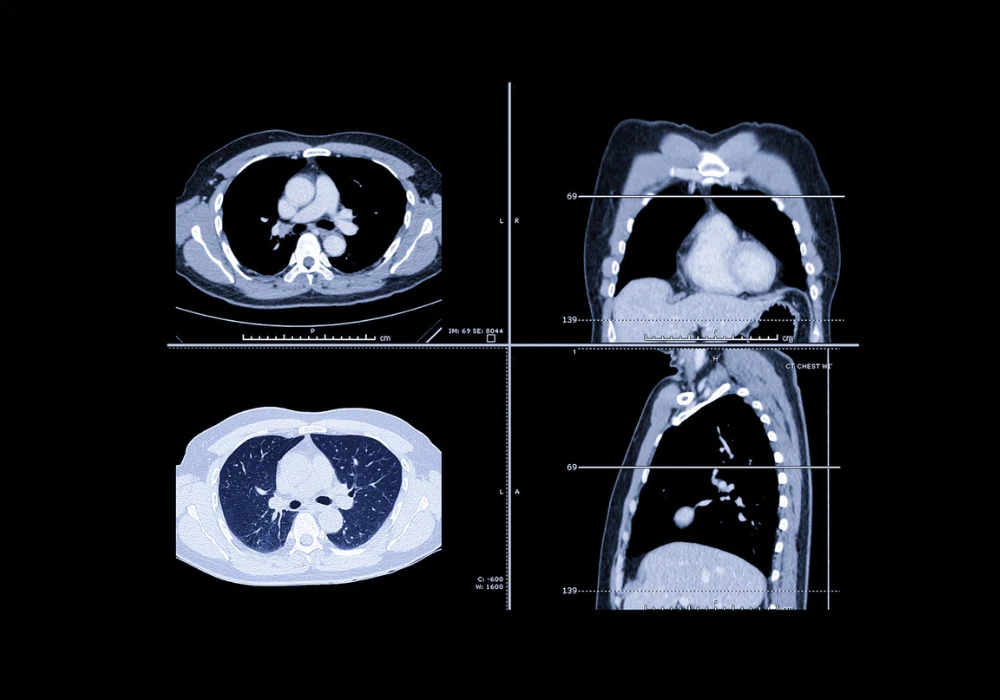
Lung cancer is the leading cause of cancer mortality worldwide, primarily due to late-stage diagnoses. The introduction of low-dose computed tomography (LDCT) screening has proven effective in detecting lung cancer at earlier stages, significantly improving survival rates. Targeting high-risk populations—particularly current or former smokers—screening with LDCT has been validated through major studies such as NLST and NELSON. However, for LDCT screening to reach its full potential, precise and standardised pulmonary nodule management is essential. To this end, the European Society of Thoracic Imaging (ESTI) has proposed a refined classification system for screen results and recommendations for managing pulmonary nodules, aiming to balance early cancer detection with minimised overdiagnosis and unnecessary follow-up.
Risk Categories and Assessment Approaches
The ESTI proposal classifies screen-detected nodules into three risk categories: negative, indeterminate and positive. Negative results represent very low malignancy risk and typically comprise the majority of cases, allowing for annual follow-up. Indeterminate nodules, with low to intermediate risk, require closer monitoring at intervals of 1 to 6 months. Positive results indicate a high probability of cancer, necessitating diagnostic work-up and multidisciplinary team discussion.
Must Read: MRI Versus LDCT in Long-Term Nodule Follow-Up for COPD Patients
Three major classification approaches are outlined. The first is morphology-based, where nodules are assigned categories based on predefined structural characteristics. An example is Lung-RADS v2022, which assigns nodules to categories based on size and density. The second is model-based, using tools like the Brock model that incorporate clinical and demographic data alongside imaging features to estimate malignancy risk. The third approach involves deep learning-based models that analyse imaging data directly to calculate malignancy probability. While not yet integrated into official protocols, these AI-based tools are being prospectively evaluated and may eventually reduce interpretation variability and enhance accuracy.
Morphological and Volumetric Criteria
Morphological indicators remain central to malignancy assessment. Features such as spiculation, perinodular architectural distortion, pleural indentation and bubble-like lucencies are considered suggestive but not definitive signs of malignancy. For subsolid nodules, a solid component measuring 5 mm or more is generally interpreted as invasive, although exceptions exist, including invasive cancers that appear as pure ground-glass nodules.
Volumetric assessment, particularly measuring volume doubling time (VDT), is increasingly preferred over manual diameter measurements due to its superior precision. A 100% increase in volume corresponds to only a 26% increase in diameter, making subtle growth more detectable with volumetry. It also captures asymmetric growth, especially in the z-axis, that diameter-based methods may miss. Nevertheless, volumetry has limitations, especially when segmenting subsolid or cystic nodules, or those adjacent to anatomical structures like the pleura or blood vessels. Factors such as scan reconstruction parameters, patient positioning and respiratory motion can also affect volume measurement reliability. Consistency in software and scanning technique is thus critical to ensuring accurate growth assessment.
Recommendations for Screening and Follow-up
The ESTI guidelines recommend specific flowcharts for managing solid and subsolid nodules detected at baseline, during follow-up and when newly observed. At baseline, nodules are classified by morphology, density and size. Nodules with benign features such as calcifications or typical intrapulmonary lymph node morphology are considered negative and managed with annual screening. Nodules showing suspicious features or exceeding certain volume or diameter thresholds enter the indeterminate or positive categories and require short-term follow-up or diagnostic work-up.
For prevalent nodules, growth is assessed using VDT or diameter change, with defined thresholds at three, six and twelve months. Manual measurements are used when volumetry fails, with an increase of more than 1.5 mm in diameter considered substantial. Subsolid nodules with solid components exceeding 80% of the total diameter are reclassified as solid nodules. Structured reporting is recommended to ensure clarity and reduce interpretation discrepancies.
Artificial intelligence and computer-aided detection tools are expected to play a larger role in the future. Over fifteen CE-certified AI algorithms are currently available in Europe, though few are reimbursed. These systems, particularly those based on deep learning, have shown strong potential in malignancy risk estimation and may improve identification of aggressive tumours while reducing follow-up for benign lesions. Radiomics and AI-driven methods to differentiate between preinvasive and invasive adenocarcinoma are also under development, although radiologist measurements of the solid component still outperform these models in many cases.
The ESTI nodule management recommendations offer a systematic and practical approach to interpreting LDCT lung cancer screening results. By focusing on lesion aggressiveness, volumetric growth and morphological features, the guidelines aim to reduce false positives and minimise unnecessary interventions without compromising early cancer detection. Structured reporting and standardised protocols improve communication and diagnostic accuracy. While promising AI-based tools are emerging, further prospective validation is needed before they can be routinely integrated into clinical workflows. The ongoing implementation and refinement of these recommendations will be crucial in optimising lung cancer screening across Europe.
Source: European Radiology
Image Credit: iStock