Identifying aggressive prostate cancer before treatment is essential yet difficult. Biopsy remains the reference for grading but samples a small part of the tumour and can miss areas that drive outcomes. Multiparametric MRI (mpMRI) offers a noninvasive view of the prostate, though traditional summaries can smooth over important differences within the same lesion. An approach called habitat imaging uses the rich detail of mpMRI to divide a tumour into subregions that behave differently. Using radical prostatectomy pathology as a benchmark, researchers assessed whether these habitats can flag high-grade disease more reliably than clinical imaging factors alone and whether combining both streams of information improves preoperative risk assessment across multiple hospitals.
Multicentre Cohorts and Imaging Framework
The analysis covered 359 men with prostate cancer scanned before surgery between January 2018 and June 2024. They were split into a training group, an internal test group from the same centre and an external test group from three other hospitals. Pathology after surgery assigned International Society of Urological Pathology (ISUP) grades, grouped into low to medium (ISUP ≤ 3) and high grade (ISUP ≥ 4). The split across the entire cohort was broadly even, ensuring that findings reflected a mix of risk levels.
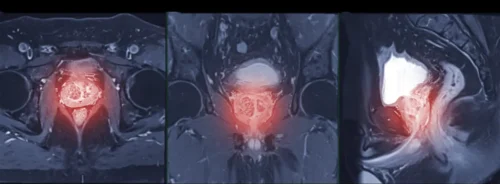
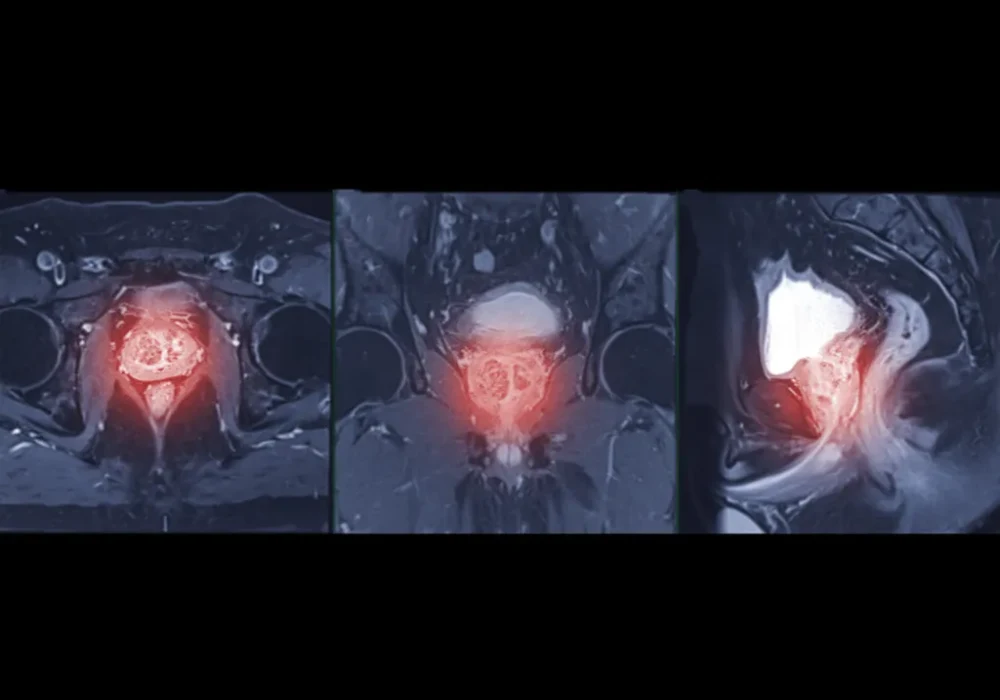
Imaging included standard components of mpMRI. Maps derived from diffusion and perfusion sequences were prepared with consistent preprocessing and registration to limit site effects. Radiologists outlined lesions, avoiding areas such as necrosis or haemorrhage, and transferred the same outlines across all maps. A simple, reproducible clustering step then grouped voxels into three habitats based on their diffusion and perfusion characteristics. For each lesion, the resulting habitat map showed where each subregion lay and how much space it occupied, turning complex images into a concise, interpretable profile that could be compared with surgical pathology.
Must Read: Reducing Unnecessary Biopsies in Prostate Cancer Detection
To ensure measurements were dependable, the team reported strong agreement between readers for key clinical-imaging factors, including maximum tumour diameter and the Prostate Imaging Reporting and Data System (PI-RADS) v2.1 score. Because two habitat proportions were strongly negatively linked, only the proportion of one key habitat was taken forward to multivariable modelling to avoid redundancy.
What the Habitats Revealed
Three distinct habitats emerged repeatedly across lesions. One subregion, referred to here as Habitat 1, showed the combination of more restricted diffusion and greater structural complexity on the MRI-derived maps. Another, Habitat 2, tended to display the opposite pattern, while Habitat 3 sat between them. When these habitat maps were aligned with post-surgical pathology, a clear pattern appeared. High-grade cancers contained a larger share of Habitat 1, whereas low to medium grade cancers were more commonly composed of Habitat 2.
This link between anatomy on mpMRI and pathology after surgery is clinically meaningful. Rather than relying on a single average value for a whole lesion, habitat imaging preserved intratumoural differences and connected them to grade. In the training data, Habitat 1 correctly identified high-grade disease in the great majority of relevant cases. Although the other subregions carried useful context, their association with high-grade pathology was weaker. These observations supported using the proportion of Habitat 1 as a concise imaging marker of aggressiveness that could be combined with routine clinical-imaging information.
Importantly, the habitat maps remained grounded in images that clinicians already use. The process did not demand exotic acquisition or opaque modelling. It repackaged familiar sequences into a spatial summary that highlighted the parts of a tumour most likely to host high-grade disease, providing a visual cue that complements radiologist assessment and could inform targeted sampling or treatment planning.
Building a Practical Prediction Tool
Two preoperative models were built with surgical pathology as the reference. The first, a clinical imaging model, used prostate-specific antigen (PSA), maximum tumour diameter and PI-RADS score. The second, called the habitat imaging–clinical imaging (HICI) model, added the proportion of Habitat 1. In univariable analysis, clinical T stage, PSA, maximum tumour diameter, PI-RADS and the proportion of Habitat 1 were linked to high-grade disease. Multivariable analysis retained PSA, maximum tumour diameter, PI-RADS and the proportion of Habitat 1 as independent contributors, underscoring that the habitat signal adds information rather than echoing what clinicians already know.
Performance favoured the combined approach. In the training cohort, HICI achieved a higher area under the receiver operating characteristic curve than the clinical imaging model. The advantage held in external validation, where HICI again outperformed the baseline. In the internal test set, the difference was smaller but directionally similar. Beyond summary discrimination, the combined model delivered gains in sensitivity and overall balance of errors, with a slight trade-off in specificity in the external cohort. Calibration and decision curve analyses supported a better net clinical benefit for HICI, indicating that, across reasonable decision thresholds, incorporating habitat proportion would likely reduce missed high-grade cases without an undue rise in false positives.
Crucially, the HICI model remained straightforward. It blended widely available clinical-imaging inputs with a single additional MRI-derived proportion. That design keeps implementation practical while retaining interpretability: clinicians can see both the overall risk estimate and the map highlighting where the aggressive habitat sits within the lesion.
By capturing spatial heterogeneity that whole-lesion summaries can miss, mpMRI habitat imaging linked a distinct subregion to high-grade prostate cancer and improved preoperative grading when combined with PSA, maximum tumour diameter and PI-RADS. The combined HICI model showed stronger discrimination and clinical utility across centres than clinical imaging alone, while remaining based on familiar imaging and a simple proportional measure. Although the work was retrospective with manual segmentation and restricted to men proceeding to surgery, multicentre testing supports generalisability. Habitat mapping offers an accessible way to refine risk assessment before treatment, aligning intervention more closely with the underlying biology reflected in the MRI signal.
Source: Radiology: Imaging Cancer
Image Credit: iStock