Breast cancer (BC) remains the most prevalent malignancy among women globally. The classification and accurate identification of human epidermal growth factor receptor 2 (HER2) status are critical for determining appropriate treatment plans, particularly for targeted therapies. Traditionally, HER2 status is assessed through invasive methods such as immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). However, these techniques can be costly, time-consuming, and prone to sampling errors due to the heterogeneous nature of tumours. Recent advances in imaging technology, specifically magnetic resonance imaging (MRI) and radiomics, have opened up new avenues for non-invasive prediction of HER2 status. A recent study in Academic Radiology discusses the findings from a study that developed and compared predictive models based on conventional MRI (cMRI) and radiomics features, highlighting the superior performance of a comprehensive model that integrates both methods.
MRI and Radiomics in HER2 Status Prediction
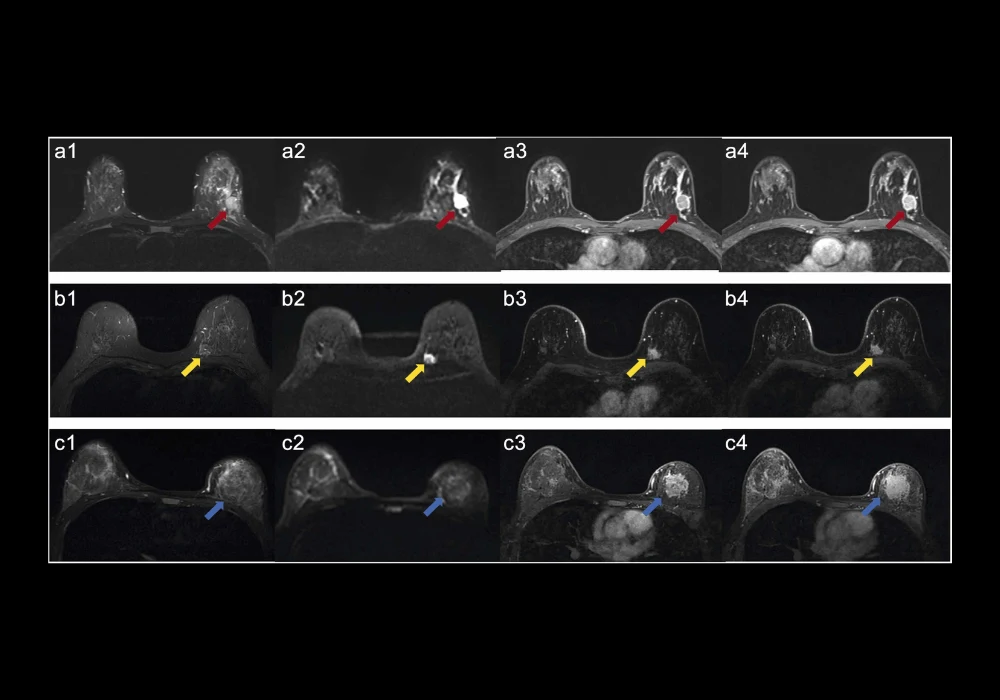
MRI has long been a valuable tool in the diagnostic process of breast cancer, offering detailed anatomical and functional imaging. Radiomics, a technique that extracts a large number of features from medical images, can provide additional insights into tumour heterogeneity and molecular characteristics. Previous studies have demonstrated the potential of MRI-based radiomics models to differentiate between various HER2 statuses in breast cancer. For instance, radiomics features from different MRI sequences have been used to distinguish HER2-zero, HER2-low, and HER2-positive tumours. However, most of these models relied solely on radiomics features and did not incorporate conventional MRI features, potentially limiting their predictive power.
The study in focus addressed this gap by developing a comprehensive model that combined both cMRI and radiomics features. The cMRI features included parameters such as tumour size, shape, and enhancement patterns. In contrast, radiomics features were derived from multiple MRI sequences, including T2-weighted imaging (T2WI) and dynamic contrast-enhanced MRI (DCE-MRI). By integrating these two approaches, the researchers aimed to improve the accuracy of HER2 status prediction, ultimately aiding in better treatment planning for patients with breast cancer.
Comparative Performance of Predictive Models
The study compared three distinct models for predicting HER2 status: a cMRI-based model, a radiomics-based model, and a comprehensive model that combined both cMRI and radiomics features. The performance of these models was evaluated using metrics such as the area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and accuracy. The results revealed that the comprehensive model outperformed both the cMRI and radiomics models across all metrics.
In the training set, the comprehensive model achieved an AUC of 0.859, compared to 0.705 for the cMRI model and 0.819 for the radiomics model. Similar trends were observed in the test set, where the comprehensive model's AUC was 0.842, significantly higher than the AUCs of the cMRI (0.639) and radiomics (0.797) models. The superior performance of the comprehensive model was further validated through the DeLong test, which confirmed that the combination of cMRI and radiomics features provided a significant improvement over using radiomics features alone.
These findings underscore the importance of combining multiple imaging modalities and techniques to capture the full spectrum of tumour characteristics. While radiomics can offer detailed information on tumour heterogeneity, cMRI provides crucial anatomical and functional context, making the comprehensive model a more robust tool for predicting HER2 status.
Clinical Implications and Future Directions
The ability to noninvasively predict HER2 status using a comprehensive MRI-radiomics model has significant clinical implications. For one, it could reduce the need for invasive biopsy procedures, particularly in cases where tissue samples are difficult to obtain or where tumours exhibit significant heterogeneity. Additionally, the model could aid in identifying patients who are eligible for HER2-targeted therapies, including those with HER2-low expression, a subset of breast cancer that has recently emerged as a targetable category.
Moreover, using such models in clinical practice could lead to more personalised treatment plans, improving patient outcomes and reducing the risk of overtreatment or undertreatment. However, further validation is needed through prospective, multicentre studies with larger cohorts before this model can be widely implemented. Additionally, incorporating deep learning algorithms could enhance the model's predictive power by extracting more abstract and informative features from MRI images.
Conclusion
The study demonstrated that a comprehensive model integrating conventional MRI and radiomics features outperforms single-modality models in noninvasively predicting HER2 status in breast cancer. This finding highlights the potential of combining advanced imaging techniques to improve diagnostic accuracy and treatment planning for patients with breast cancer. While further research and validation are necessary, the comprehensive model represents a promising step toward more personalised and non-invasive cancer care. As technology continues to evolve, integrating radiomics with other imaging modalities and machine learning techniques will likely become a standard approach in cancer diagnostics and treatment planning.
Source & Image Credit: Academic Radiology