ICU Management & Practice, Volume 24 - Issue 4, 2024
Early mobilisation includes several progressive kinds of movements. Many barriers and safety concerns must be addressed to allow a smooth and effective introduction of this procedure in the ICU daily practice.
Introduction
Early mobilisation is recommended as part of a multi-component, nonpharmacological strategy to improve physical, mental and cognitive outcomes of critically ill adults. Physical rehabilitation minimises muscle weakness and impaired physical functioning, reduces cognitive impairment and optimises nocturnal sleep (Devlin et al. 2018). Early mobilisation (EM) in the ICU includes passive and active movement and training. This multifaceted intervention involves a wide range of activities, from in-bed to out-of-bed exercises, as shown in Table 1 (Clarissa et al. 2019; Nydahl et al. 2023; Watanabe et al. 2022).
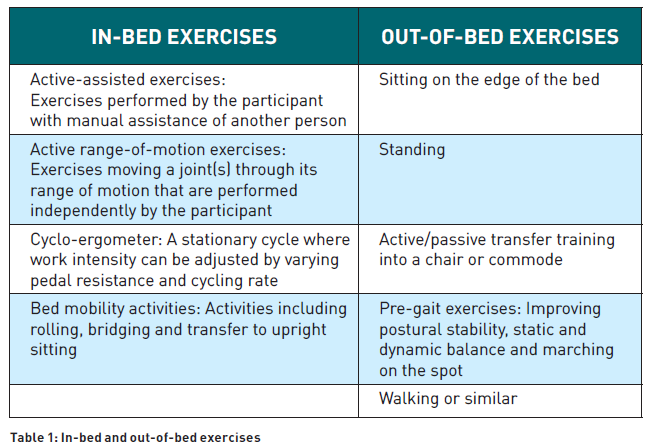
EM also refers to any other type of active exercise modality started while the participant is in the ICU, like activities of daily living (ADLs): self‐care tasks such as eating, bathing, dressing and toileting (Doiron et al. 2018). Some studies exclude from this definition interventions such as turning in bed, change of positions, particularly when done to prevent pressure sores, or use of neuromuscular electrical stimulation or robotics (Nydahl et al. 2023), whereas other studies include them (Doiron et al. 2018). At present, EM lacks a specific definition and encompasses a range of heterogeneous interventions that have been used alone or in combination (Hodgson et al. 2013).
The combination of critical illness and prolonged immobility results in substantial muscle wasting during the ICU stay. That’s one reason why EM should start as soon as feasible after admission to the ICU. Evidence suggests that starting rehabilitation within 72 hours from admission may lead to improvements in both physical and cognitive function, minimising the sequelae of prolonged physical immobilisation during mechanical ventilation, such as muscle atrophy, weakness, and paresis, thereby enhancing future autonomy and quality of life (Doironet al. 2018; Matsuoka et al. 2023). Furthermore, EM is a holistic activity with physical, cognitive, and psychosocial dimensions, including coordinated movements, increased proprioception, gravity effects, sympathetic activation of neurotransmitters, improved cerebral perfusion, cognitive activation and participation, and interaction with the environment and healthcare providers (Lai et al. 2017). These aspects may contribute to improving a patient’s orientation and overall well-being, possibly facilitating their return to functional independence (Patel et al. 2023; Zhang et al. 2019).
The most common protocol was created by Morris et al. (2008) and is divided into four levels:
- Level I: Passive extremities movements for unconscious patients.
- Level II: Active extremities movements and interaction with the physical therapist for conscious patients who can respond to simple commands in a sitting position on the bed.
- Level III: Like level II but sitting on the edge of the bed for patient's biceps strength of >3/5 on the Medical Research Council Scale (Medical Research Council 1976).
- Level IV: Like level II, but with the patient actively moving from the bed to a chair beside the bed for patient's quadriceps strength of >3/5.
The highest level of mobilisation is kept for as long as possible before a step-down to lower levels of activity, should the patient become fatigued, as measured by the ICU Mobility Scale (Hodgson et al. 2014; Lai et al. 2017).
EM should be applied in short and frequent sessions (Eggmann et al. 2022). Morris suggests twice daily, five days a week and, if possible, involving caregivers (Lai et al. 2017; Morris et al. 2008).
The sessions are individually tailored to achieve the highest possible level of mobilisation that is deemed to be safe for the patient at the initiation of daily therapy. EM should be integrated into a patient-centred approach (Zhang et al. 2019).
Implementing an EM programme requires a multidisciplinary team and approach: a critical care nurse, nursing assistant, respiratory therapist, physical therapist, and even family (Lai et al. 2017). EM can be delivered either as a standalone intervention or as part of a broader care approach, such as the ABCDEF bundle, which addresses analgesia, sedation, delirium, mobilisation, and family integration (Frade-Mera et al. 2022; Nydahl et al. 2023).
Barriers to Implementation of Early Mobilisation
Implementation of EM in the ICU can be difficult due to several factors. Barriers correlated with EM could be divided into four groups: patient-related, structural, cultural and process-related barriers (Alaparthi et al. 2020).
Patient-related barriers
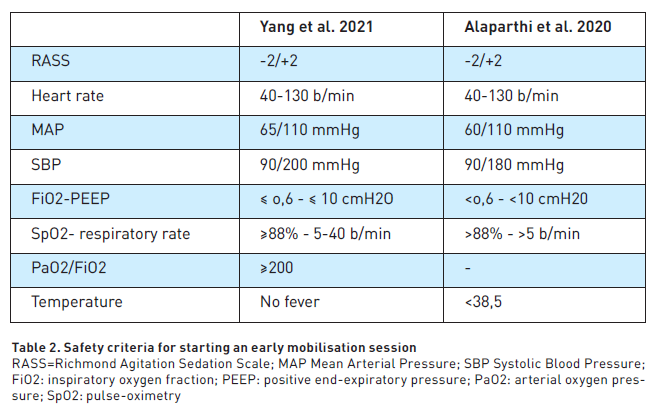
These include haemodynamic instability, pain, deep sedation, agitation and delirium, patient denial, lack of motivation, and lack of intensive care unit equipment and devices. Interventions must be tailored to patient conditions such as level of arousal, haemodynamic stability and tolerance. Fontela et al. (2018) reported in their multicentre Brazilian survey that the most common barriers in the application of EM were weakness, haemodynamic instability and sedation. Nurse’s opinions about factors limiting EM were analysed in two surveys. In the cross-sectional multicentre survey of Zhang et al. (2022), instability of patients (94.9%), mechanical ventilation (84.6%) and unconsciousness (82.8%) were perceived as the main barriers. In a survey by Babazadeh et al. (2021), deep sedation (88.9%), mobilisation of obese patients (82.2%), mobilisation of agitated patients (65%) and pain induced by mobilisation of mechanically ventilated patients (57.9%) were perceived as significant barriers. Physiotherapists identified haemodynamic instability, raised intracranial pressure, low platelet count and mental instability as barriers (Tadyanemhanduet al. 2022). Barriers to EM were more frequent in the first seven days after admission (Watanabe et al. 2021); haemodynamic instability was the most common barrier on day 1 and day 2, while a reduced level of consciousness was most common on day 3 to 5 (Watanabe et al. 2021). Safety criteria for EM have been proposed in Table 2.
Structural barriers
These include limited staff, lack of guidelines, lack of equipment and lack of protocols. ICU staff reported that there is insufficient equipment and staff (87.9%), lack of appropriate training (83.6%) and lack of time for mobilising patients (Zhang et al. 2019; Babazadeh et al. 2021; Akhtar et al. 2021). Work experience is an important aspect for the perception of the barriers: health professionals with years of experience in hospitalised patients have a better approach to early mobilisation (Tadyanemhandu et al. 2022; Goodson et al. 2020). The lack of guidelines is remedied by the use of their own experience (Goodson et al. 2020). On the other hand, the introduction of guidelines and protocols alone is not sufficient to promote EM (Anekwe et al. 2020; Akhtar et al. 2021).
Cultural barriers
These include lack of knowledge and awareness about benefits and feasibility of EM (Anekweet al. 2020; Akhtar et al. 2021). Nurses and physicians that did not receive education and training on EM have inadequate knowledge about it and a low level of intention to apply EM, considering EM too risky and unnecessary (Zhang et al. 2022; Anekwe et al. 2020;Tadyanemhandu et al. 2022).
Process-related barriers
These include a lack of daily coordination and planning and risks for mobility providers. Patient safety and medical disputes are something nurses are concerned about (Zhang et al. 2022). Poor coordination in the multidisciplinary group can cause problems in planning daily treatments without goal-sharing (Anekwe et al. 2020; Tadyanemhanduet al. 2022; Akhtar et al. 2021).
All these barriers must be addressed and solved to allow the adoption of EM with the right protocol and the right “dose” for all suitable patients.
Adverse Events During Early Mobilisation
Traditionally, EM was avoided for lack of awareness of its beneficial effects and for the possible adverse events which may occur to frail ICU patients. In recent years, some studies have evaluated the incidence and type of adverse events during EM. Doiron et al. (2018) published a review focused on the safety profile of EM. Overall, the analysed studies included 690 adult patients and a wide range of interventions ranging from in-bed mobility to ambulation. Among the four included studies in the review, only two reported adverse events in the intervention group that were deemed to be related to EM: one asymptomatic bradycardia, one episode of severe oxygen desaturation and one episode of catheter dislodgement. Furthermore, only 19 sessions had to be ceased due to patient instability. (Doiron et al. 2018).
In a systematic review and meta-analysis, Takaoka et al. (2020) investigated the impact of in-bed leg cycle ergometry in the ICU. They collected data from 12 RCTs and two nonrandomised studies published between 2014 and 2019. Only five adverse events were reported out of 3117 sessions (0.16%). Six of the evaluated studies reported 18 session terminations during 1829 (0.98%) cycling sessions due to complications. However, the authors underlined the heterogeneity in the definitions of adverse events and in the criteria adopted for suspending a session.
In a meta-analysis on EM in mechanically ventilated patients (Klem et al. 2021), which included 17 studies and 1805 patients, only two life-threatening adverse events were identified: a case of bradycardia and one of hypoxia. A total of 79 adverse events were reported during 5675 sessions (1.4 %) and, among them, 35 of these events caused the interruption of the sessions.
Two studies evaluated EM safety in patients undergoing continuous renal replacement therapy (CRRT) or during extra-corporeal life support (ECLS). Wang and colleagues (2014) published a prospective study, collecting data from 33 patients admitted to two Australian ICUs. The primary outcome of this study was to investigate the safety of mobilisation in patients who underwent CRRT via femoral vascular access. The authors included, as adverse events, the following: catheter dislodgement, clotting or disruption of filter and lines, bleeding or haematoma, clinical suspicion of thrombosis and arrhythmias. They tested three levels of mobilisation: in-bed passive mobilisation, sitting on the edge of the bed and walking. Each planned activity lasted 20 minutes. No adverse events occurred during mobilisation or after it. One of the participants also had a Swan-Ganz catheter in place, but neither arrhythmias nor other relevant clinical sequelae were reported. During mobilisation, no CRRT machine alarms rang. The authors also tested the hypothesis that mobilisation might reduce circuit and filter clotting. Data collected from the femoral venous access subgroup indicate that passive hip flexion and position changes might have increased filter life (Wang et al. 2014).
Most of the patients requiring ECLS are still treated with cautious strategies that include deep sedation and invasive mechanical ventilation since immobilisation and reduced range of passive movements may minimise complications. In 2023, Cucchi and co-authors performed a systematic review in order to provide evidence-based recommendations on early mobilisation in awake patients undergoing ECLS (Cucchi et al. 2023). They summarised data from 29 observational studies and one RCT, including 1157 patients who received physiotherapy while undergoing veno-venous or veno-arterial extracorporeal support. They investigated the incidence of adverse events caused by mobilisation while on ECLS, such as circuit kinking or cannula dislocation, bleeding, haemodynamic instability, respiratory failure or need for tracheal intubation, neurological deterioration or infections. Patients supported with non-invasive ventilation (NIV) and with femoral cannulation were more likely to develop mechanical and haemorrhagic complications (respectively 4.2% and 4.4%). Infective and cardiovascular complications were mostly reported in patients undergoing veno-arterial ECLS (11.3 and 9.5%). Neurological sequelae were rare and mostly affected patients supported with NIV who could walk (7.8%). They concluded that EM, and even ambulation, can be safely performed regardless of the cannulation site (Cucchi et al. 2023).
Conclusion
Despite the potential benefits of EM, barriers to its implementation have been reported. Further research is needed to standardise practices and determine optimal initiation timing and extent of mobilisation, including considerations on duration, intensity, and frequency in order to maximise its effectiveness and minimise adverse events.
Conflict of Interest
None.
References:
Akhtar PM et al. (2021) Knowledge, attitudes, and perceived barriers of healthcare providers toward early mobilization of adult critically ill patients in intensive care unit. Indian J Crit Care Med. 25(5):512–518.
Alaparthi GK et al. (2020) Effectiveness, safety, and barriers to early mobilization in the intensive care unit. Crit Care Res Prac. 7840743.
Anekwe DE et al. (2020) Intensive care unit clinicians identify many barriers to, and facilitators of, early mobilisation: a qualitative study using the Theoretical Domains Framework. J Physiother. 66(2):120–127.
Babazadeh M et al. (2021) Perceived barriers to early mobilization of intensive care unit patients by nurses in hospitals affiliated to Jundishapur University of Medical Sciences of Ahvaz in 2019. J Med Life. 14(1):100–104.
Clarissa C et al. (2019) Early mobilisation in mechanically ventilated patients: a systematic integrative review of definitions and activities. J Intensive Care. 7(1).
Cucchi M et al. (2023) Awake extracorporeal life support and physiotherapy in adult patients: A systematic review of the literature. Perfusion. 38(5):939-958
Devlin JW et al. (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 46(9):e825–e873.
Doiron KA et al. (2018) Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database of Syst Rev. 3(3):CD010754.
Eggmann S et al. (2022) Cardiorespiratory response to early rehabilitation in critically ill adults: A secondary analysis of a randomised controlled trial. PloS One. 17(2):e0262779.
Fontela PC et al. (2018) Early mobilization practices of mechanically ventilated patients: A 1-day point-prevalence study in southern Brazil. Clinics. 73:e241.
Goodson CM et al. (2020) Perceived barriers to mobility in a medical ICU: The patient mobilization attitudes & beliefs survey for the ICU. J Intensive Care Med. 35(10):1026–1031.
Hodgson C et al. (2013) Clinical review: early patient mobilization in the ICU. Crit Care. 17(1):207.
Hodgson C et al. (2014) Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 43(1):19–24.
Klem HE et al. (2021)Early activity in mechanically ventilated patients - a meta-analysis.Tidsskr Nor Laegeforen. 141(8).
Lai CC et al. (2017) Early mobilization reduces duration of mechanical ventilation and intensive care unit stay in patients with acute respiratory failure. Arch Phys Med Rehabil. 98(5):931–939.
Medical Research Council (1976) Aids to examination of the peripheral nervous system. Memorandum n. 45. London: Her Majesty’s Stationary Office.
Morris PE et al. (2008) Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 36:2238-2243.
Nydahl P et al. (2023) Early mobilisation for prevention and treatment of delirium in critically ill patients: Systematic review and meta-analysis. Intensive Crit Care Nurs. 74:103334.
Patel BK et al. (2023) Effect of early mobilisation on long-term cognitive impairment in critical illness in the USA: a randomised controlled trial. Lancet Respir Med. 11(6):563–572.
Tadyanemhandu C et al. (2022) Barriers and facilitators to implementation of early mobilisation of critically ill patients in Zimbabwean and South African public sector hospitals: a qualitative study. Disabil Rehabil. 44(22):6699–6709.
Takaoka A et al. (2020) The efficacy and safety of in-intensive care unit leg-cycle ergometry in critically ill adults. A systematic review and meta-analysis. Ann Am Thorac Soc. 17(10):1289-1307.
Watanabe S et al. (2022) Association between early mobilization in the ICU and psychiatric symptoms after surviving a critical illness: A multi-center prospective cohort study. JClin Med. 11(9):2587.
Yang R et al. (2021) Safety assessment criteria for early active mobilization in mechanically ventilated ICU subjects. Respir Care. 66(2):307–315.
Zhang L et al. (2019) Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PloSOne. 14(10):e0223185
Zhang H et al. (2022) Early mobilization implementation for critically ill patients: A cross-sectional multi-center survey about knowledge, attitudes, and perceptions of critical care nurses. Int J Nurs Sci. 9(1):49–55.










