ICU Management & Practice, Volume 24 - Issue 4, 2024
A literature review to highlight how early mobilisation can improve patient-important outcomes, including length of ICU and hospital stay, duration of mechanical ventilation and overall quality of life in ICU survivors and the risks associated with EM and barriers to safe implementation of current practices, future directions, and the need for more studies to identify effective early mobilisation protocols.
Introduction
An estimated 13 to 20 million people annually require life support in intensive care units (ICU) worldwide (Adhikari et al. 2010). Among those patients who require mechanical ventilation, 25% will develop prolonged neuromuscular weakness (Ali et al. 2009; De Jonghe et al. 2002). ICU-acquired weakness (ICUAW) is defined as clinically detected diffuse and symmetric muscle weakness without any cause other than the critical illness itself (Kress and Hall 2014). ICUAW has been shown in various studies to increase the risk of death, prolong hospitalisation, and impair recovery (Van Aerde et al. 2020; Hermans et al. 2014). Amongst causative factors, immobilisation and disuse are considered important contributors to the development of ICUAW. The concept of early mobilisation (EM) of critically ill patients has gained substantial favour due to its numerous benefits in patient recovery. Traditionally, critically ill patients were kept immobile to prevent complications, but more recent research has shown that engaging patients in physical activity as soon as it is clinically feasible helps to reduce muscle atrophy, improves cardiovascular function and improves overall functional outcomes in ICU survivors. EM has been linked to shorter hospital length of stay (LOS), reduced incidence of delirium, and better long-term physical and cognitive functioning. This proactive approach represents a paradigm shift towards more dynamic and patient-centred care in critical care settings.
Early mobilisation of ICU patients presents its own unique challenges. These include identifying the patient population that meets the specific criteria for diagnosis of ICUAW, formalisation of clinical tests used to identify ICUAW, screening of patients who will most benefit from early mobilisation, the actual techniques used to carry out early mobilisation, and lastly, the safety of such interventions. Critically ill patients typically have poor cardiopulmonary reserve, often require heavy sedation, and are bound by medical devices and equipment (lines, tubes, mechanical ventilation, and monitors), accidental dislodgement of such which can be fatal. Finally, these interventions should ideally show improvement in patient-centred outcomes, including mortality/morbidity benefits and/or an improvement in overall quality of life.
We reviewed the available literature to assess current knowledge of EM in critically ill patients. The term “early mobilisation” remains ill-defined and encompasses a range of heterogeneous interventions that have been used alone or in combination.Nevertheless, several studies suggest that different forms of EM may be both safe and feasible in ICU patients, including those receiving mechanical ventilation (Kress et al. 2000; Schaller et al. 2016). Unfortunately, studies of EM are primarily single-centre in origin, may have limited external validity and have highly variable control arms. Additionally, emerging technologies such as cycle ergometry, transcutaneous electrical muscle stimulation and video therapy are increasingly being used to achieve EM despite limited evidence of efficacy. Although evidence suggests that EM in the ICU is safe, feasible, and beneficial, it is also labour-intensive and requires appropriate staffing and equipment. Further research is required to identify specific patient populations, techniques, efficacy, and structured algorithms to maximise the benefit and safety of EM while not creating unnecessary demand on already taxed ICU staff and burdensome workflows.
Background
Historically, bedrest was considered a treatment for critical illness. In 1899, it was discovered that bedrest was deleterious in the post-operative period and that LOS could be shortened from days or weeks to hours by instituting earlier mobility (Ries 1899).In the late twentieth century, emerging evidence demonstrated that continuous sedation was associated with prolonged duration of mechanical ventilation as well as longer ICU and hospital LOS.After this, a landmark study by Kress et al. (2000)showed that daily interruption of sedation led to decreased duration of mechanical ventilation and ICU LOS. Researchers then began to examine the effect of mobilising ICU patients. Landmark studies began to show that early mobility decreased ICU and hospital stay, with patients returning earlier to independent functional status with significantly less post-ICU delirium (Bailey et al. 2007).
Early mobilisation is the application and intensification of physical rehabilitation given to patients within the initial two to five days of critical illness. It is delivered more regularly than conventional practice, which typically consists of passive range of motion exercises, reserving active mobilisation for the post-acute phase of illness. By the 1970s, the advantages of early mobilisation in mechanically ventilated patients were studied in adults (Burns and Jones 1975).Burns and Jones described the use of a novel device easily assembled from commercially available parts to incorporate a stable-wheeled walker with an armrest, respirator, oxygen source and IV pole. It demonstrated the utility of early ambulation to facilitate weaning and address the problems associated with prolonged rest. Since the early nineteenth century, studies have shown that EM of critically ill patients reduces the incidence of ICUAW, improves functional capacity, decreases days of mechanical ventilation and length of ICU stay and decreases comorbidities like development of deep venous thrombosis, ventilator-associated pneumonia, and integumentary pressure injuries (Zhang et al. 2019; Zang et al. 2019). However, these studies have been limited by small sample size and lack of standardisation in the population, intervention, and outcome measures. Most importantly, there are significant discrepancies between the diagnostic criteria used for ICUAW.
The Medical Research Council (MRC) sum score for muscle strength evaluation is an assessment of muscle strength and has been used to objectively describe ICUAW. The MRC sum score ranges between 0 and 60, and scores < 48 and < 36 co-relate to ICUAW and severe ICUAW, respectively (Hermans et al. 2012). Muscles tested include wrist flexion, forearm flexion, shoulder abduction, ankle dorsiflexion, knee extension, and hip flexion. Grades for each muscle are from 0 (no visible contraction) to 5 (normal strength). A total score of ≤ 48 with symmetrical weakness is diagnostic of ICU-AW after exclusion of other causes of weakness.
Certain patient populations are at a higher risk of developing ICU-AW. Nonmodifiable risk factors include older age, female sex, obesity, sepsis, and multiorgan failure.Unsurprisingly, in mechanically ventilated patients, the use of vasoactive medications and prolonged sedation has been associated independently with ICU-AW (Wolfe et al. 2018). Modifiable risk factors are extensive but include hyperglycaemia, use of steroids and immobility, especially in patients suffering from refractory hypoxaemia treated with neuromuscular blocking agents. Several observational studies of various EM interventions and their primary outcomes and findings are summarised in Table 1.
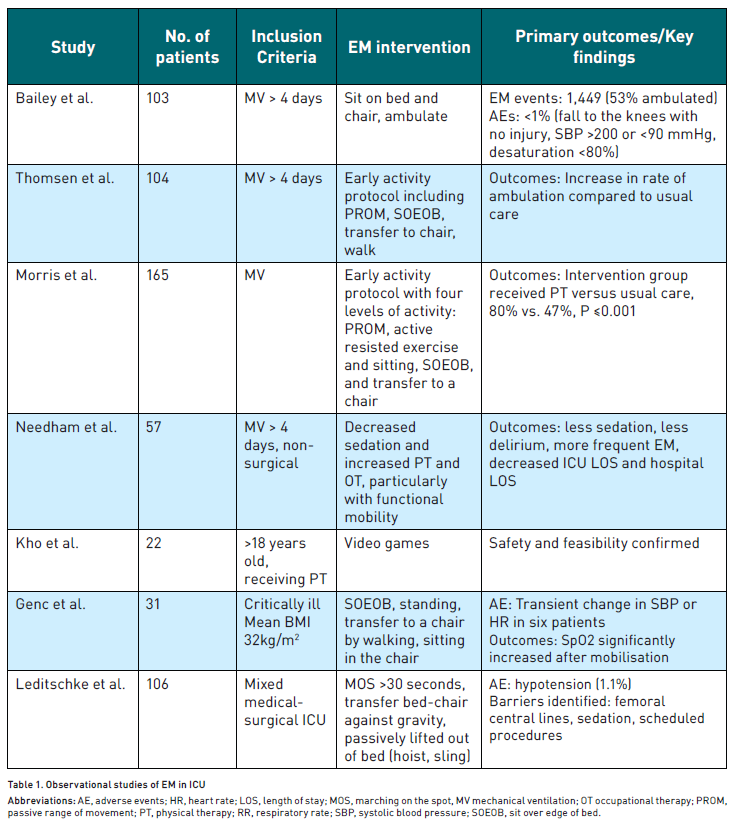
In the first observational study listed in the table, Bailey et al. (2007) documented 1,449 EM interventions in 103 patients. Of these, 53% involved ambulating patients who relied on positive pressure ventilation through an endotracheal tube or tracheostomy. Adverse events occurred in only 1% of these EM activities. This type of EM treatment utilised existing ICU staff, including nurses, technicians, physical therapists, and respiratory therapists.
Thomsen et al. (2008) conducted another study involving a before-and-after cohort study of 104 patients with respiratory failure necessitating ICU transfer. Patients under the care of an EM-focused ICU significantly increased the likelihood of ambulation during the patients' ICU stay (P <0.0001). 88% of patients survived to hospital discharge, with an average ambulation distance in the ICU of 200 feet.
Schweickert et al. (2009) conducted a prospective, outcome assessor blinded, RCT in two U.S. medical centres. This trial compared EM interventions with standard care in mechanically ventilated patients expected to have prolonged ventilated. The EM protocol included progressive activities during sedation interruption, leading to improved functional outcomes. This trial demonstrated the safety and feasibility of EM, highlighting its potential benefits in clinical practice.
Given the numerous benefits in these landmark studies, EM garnered significant attention. However, despite these initial successes, more recent studies have shown mixed results. In a 2016 RCT by Morris et al. (2016),standardised rehabilitation therapy compared with usual care did not demonstrate improvement in hospital LOS (primary outcome; P = 0.41) or ICU LOS (P = 0.68) or duration of mechanical ventilation (P = 0.59) but did demonstrate improved functional status at six months. Moss et al. (2016) also completed an RCT of EM in 2016 that compared an intensive PT programme with a standard-of-care PT programme in patients receiving mechanical ventilation. The intensive PT programme did not improve long-term (6-month) physical functional performance compared with the standard PT programme (primary outcome; P = .71).Notably, in the recent Treatment of Invasively Ventilated Adults with Early Activity and Mobilisation (TEAM) trial (2022), 9.2% of the patients in the EM intervention arm experienced an AE compared with 4.1% of patients in the usual care mobilisation group.
Discussion
In many ICUs, physical therapy only begins when patients are extubated (Mendez-Tellez et al. 2013). In contrast, early mobilisation starts within 48 hours of mechanical ventilation initiation and continues throughout ICU stay. This requires careful patient assessment and management, as well as interdisciplinary teamwork and training. There are many challenges to implementing early mobilisation interventions, which include identifying the patients that will benefit most from these practices, describing the mobility milestones in ICU, establishing protocols that have been shown to be a safe and consistent demonstration of improvement in long term consequences like overall mortality in ICU patients.
A variety of confounders explain the different outcomes among studies described in Table 1. These include variability of study populations, timing of intervention, functional status prior to the development of critical illness, and the use of different EM protocols. While most studies have been able to consistently demonstrate that EM improves physical function outcomes and hospital and ICU LOS, the effect on mortality, duration of mechanical ventilation, and quality of life outcomes remains unclear. Additionally, some of these studies may have been underpowered to demonstrate a difference in the primary outcome. Through our review of the current literature, we believe there is a signal towards improved physical function attributable to EM, a metric often cited by ICU survivors as vital to their sense of recovery after illness.
Patient selection for EM is varied across different studies. Surgical ICU patients, especially those undergoing cardiac surgery, seem to have benefitted the most from EM in terms of hospital length of stay and functional outcomes (Alaparthi et al. 2020).A systematic review by Santos et al. (2017) reported that early mobilisation in patients after cardiac surgery prevented postoperative complications, decreased length of hospital stay, and improved functional capacity when compared with no treatment. This is because EM has demonstrated enhanced oxygen transport and functional return, reducing postoperative complications and length of hospital stay. EM following surgery is beneficial because it improves ventilation, ventilation/perfusion matching, muscle strength and functional capacity.
Moradian et al. (2017) conducted a randomised controlled trial to study the effect of early mobilisation on pulmonary complications after coronary artery bypass graft (CABG) and found a lower incidence of atelectasis, pleural effusion, and improved oxygenation in the intervention group. While these are not patient-centred outcomes, anecdotally, these benefits likely reduce ICU LOS and improve end-organ perfusion.
Of course, confounders in surgical populations may include generally fewer comorbidities, less frailty, and better functional status compared to medical ICU populations. However, while most patients admitted to ICU benefit from physical therapy, there remains a need to identify patient characteristics that enable EM treatment to be prescribed and modified on an individual basis, with standardised pathways for clinical decision-making. To date, we are unaware of studies of the timing and duration of intervention to aid in the development of universal protocols. Furthermore, intensive care delivery relevant to EM is highly variable, including staffing structure, standardised practices, the use of written protocols, and the obvious barrier of over-sedation. Evidence for daily awakening and breathing trials is well documented in the literature and is outside the scope of this review, but similar principles may apply to EM. Lastly, patients need to be screened to determine their eligibility for the highest level of mobility with tailored patient-specific goals.
Proposed by Vasilevskis et al. in 2010, the ABCDE bundle is an effective strategy incorporating Awakening and Breathing coordination, Delirium monitoring/management, and Early exercise/mobility (Pun et al. 2019), aimed at improving the prognosis of mechanically ventilated patients by preventing delirium and ICU-acquired weakness (Vasilevskis et al. 2010).The implementation of the ABCDE bundle shortens the time spent on the ventilator, decreases the incidence of delirium, and increases the rate of early ambulatory mobilisation practice. Standing, walking, and gait exercises can reach higher levels of performance when whole ABCDE bundles are practiced. It is noteworthy that performing the A to D bundle is a prerequisite in order to effectively achieve early mobilisation. Moreover, tools like the ICU mobility scale (IMS) (Tipping et al. 2016) can be used by trained nurses/physical therapists when delivering EM to standardise the goal for patients. In contrast to mobility milestones (i.e. first time to stand or walk), which are commonly used as endpoints in studies of rehabilitation in the ICU, the IMS provides a sensitive 11-point scale, ranging from nothing (lying/passive exercises in bed, score of 0) to independent ambulation (score of 10). In one study, the IMS was predictive of 90-day mortality and discharge destination in an ICU population. The IMS is useful in providing a standardised method for assessing the daily highest level of mobilisation in the ICU for clinical and research purposes (Tipping et al. 2016). Zomorodi et al. (2012) tried to develop an early mobilisation protocol for patients in ICU. While some protocols were successful and decreased the length of ICU stay, we suggest that further studies with a larger sample size should be performed to establish the feasibility and efficacy of EM protocols.
Numerous barriers exist in delivering EM to patients admitted to the ICU. An exhaustive literature review in Chest outlined some of the barriers as well as proposed tactics to address them (Dubb et al. 2016). The study identified several barriers to EM, including concerns about medical stability, availability of appropriate equipment and trained staff, safety issues such as the risk of dislodging medical devices or patient falls, complications from sedation and delirium, and logistical challenges in the ICU environment.To address these obstacles, a multifaceted approach is proposed. This includes adopting a multidisciplinary team strategy involving physical therapists, nurses, and physicians to plan and execute safe mobilisation. Developing standardised protocols and guidelines based on patient condition and readiness is crucial, as is providing ongoing education to healthcare staff about the importance and techniques of EM. Implementing continuous monitoring tools to assess patient stability during mobilisation, engaging family members in the process, and employing a gradual progression approach starting with simple movements are also recommended. These strategies aim to overcome barriers and facilitate the implementation of EM programmes, potentially improving patient outcomes.
Farrand et al. (2014) performed a retrospective analysis of 100 consecutive patients who received ECMO, assessing the outcomes of those who participated in early mobilisation efforts. The study concluded that ambulation can be achieved safely and reliably in patients receiving ECMO with the help of a trained, multidisciplinary team. The study highlighted the potential advantages of early mobilisation for ECMO patients, suggesting that with appropriate protocols, more patients could benefit from active rehabilitation during their critical illness.
Perhaps the most comprehensive publication in this area is a recent systematic review of quantitative and qualitative studies that identified and evaluated factors influencing physical activity in the ICU setting (and post-ICU setting) (Parry et al. 2017). Eighty-nine papers were included with five major themes and 28 sub-themes: first, patient physical and psychological capability to perform physical activity, including delirium, sedation, motivation, weakness and anxiety; second, safety influences, including physiological stability and invasive lines; third, culture and team influences, including leadership, communication, expertise and administrative buy-in; fourth, motivation and beliefs regarding risks versus benefits; and lastly environmental influences including funding, staffing and equipment. Many of the barriers and enablers to physical activity were consistent across both qualitative and quantitative studies and geographical regions, and they supported themes established from previous research in this area. We suggest that most of these barriers can be overcome by raising general awareness about post-intensive care syndrome and the potential risks versus potential benefits of early mobilisation in the ICU. Systematic efforts to change ICU culture to prioritise early mobilisation using an interprofessional approach and multiple targeted strategies are important components of successfully implementing early mobility in clinical practice.
Emerging techniques used in EM include electrical muscle stimulation (EMS), cycle ergometry, hydrotherapy and a specialised tilt table called “the Sara Combilizer”. A review by Baron et al. (2019) suggested that neuromuscular stimulation in ICU has positive effects and is safe to use.A cycle ergometer is a stationary cycle with an automatic mechanism that can alter the amount of work performed by the patient. The cycle ergometer can be used passively (no work from the patient) or actively. Cycle ergometry has been tested in healthy subjects as part of the space research programme and has been found to preserve thigh muscle thickness during prolonged immobilisation. The method has been shown to be safe and feasible in studies during haemodialysis and in patients with chronic obstructive pulmonary disease. An RCT studied the effect of cycle ergometry in early mobilisation post-cardiac surgery and concluded that it was safe but did not show significant difference in independent physical activity (Lordello et al. 2020). Fossat et al. (2018) found that early in-bed cycling exercises and EMS for quadriceps did not cause any significant change in global muscle strength at discharge from ICU when compared to usual care. The Sara Combilizeris a combined tilt table and stretcher chair, which allows passive transfer of patients out of bed. It’s effectiveness in facilitating safe and early mobilisation found a reduction in time required for mobilisation and may be a beneficial adjunct to EM protocols. Hydrotherapy has also been studied, and it was found to be feasible and safe; however, further studies need to be done to assess its cost-effectiveness and benefits (Alaparthi et al. 2020). In a study of 410 patients receiving physical therapy (PT) in the medical ICU, 22 patients (5% of the total; 64% male; median age 52 years) participated in 42 PT sessions incorporating video games. The median number of video game sessions per patient was 1.0, with an interquartile range of 1.0-2.0. The primary reasons for using video game therapy were to improve balance (52%) and endurance (45%). The most frequently used video game activities were boxing (38%), bowling (24%), and balance board exercises (21%). Notably, 69% of these sessions occurred while patients were standing and 45% while patients were on mechanical ventilation. Throughout the 35 hours of PT treatment involving video games, no safety incidents were reported, with a 95% upper confidence limit for the safety event rate of 8.4%. The study concluded that the novel use of interactive video games as part of routine PT for critically ill patients is both feasible and appears to be safe based on this case series. The researchers suggest that video game therapy could potentially serve as a valuable complement to existing rehabilitation techniques for ICU patients (Kho et al. 2012).
These new interventions provide hope that EM techniques can be delivered safely among ventilated supine patients. However, their cost-effectiveness needs to be considered. Moreover, most of them require cumbersome staff training, and no trials have compared such interventions with a control group receiving standard care.
There remains a need to create standardised protocols and assessments using randomised controlled trials using best practices from the available trials and safety/implantation data to determine the optimal implementation of EM, with patient-centred outcomes including functional capacity and quality of life after ICU and hospital discharge. Despite the publication of safety recommendations and clinical practice guidelines, the implementation of early mobilisation remains a challenge in the ICU, particularly in the nonsurgical population. We recommend better adherence to sedation awakening trials and the development of mobilisation protocols, clinical leadership, and increased staff resources and training to effectively deliver EM techniques in ICU patients. Awareness of the deleterious effects of ICUAW is vital in engaging staff about the importance of EM. Further research is needed to understand the optimal timing, type and dose of interventions and their effect on long-term patient outcomes.
Conclusion
Early mobilisation in the ICU is currently a topic of much discussion and debate, with far-ranging implications for patients and healthcare systems. More than 15 RCTs in the past ten years, including several high-impact publications, have highlighted its importance and areas of future work. There are currently several international practice guidelines available and early mobilisation has been shown to be safe and feasible. There is no doubt that this intervention shows exciting potential. However, medical research has demonstrated that the results of pilot studies and observational studies may not result in improved patient-centred outcomes when tested in a larger trial. Future research should address gaps related to patient selection, dosage, team culture, and expertise. Future clinical practice guidelines in this area should focus on the engagement of patients and families in the development process and the provision of resources to support implementation based on the consideration of known barriers and facilitators. Effective and efficient EM practices require more standardised safety criteria, patient selection, protocolised approach, collaborative teamwork, specifically trained staff and patient and family engagement, as well as well-defined outcome measurements as key components of implementation.
Conflict of Interest
None.
References:
Abrams D, Javidfar J, Farrand E et al. (2014) Early mobilisation of patients receiving extracorporeal membrane oxygenation: A retrospective cohort study. Crit Care. 18:R38.
Adhikari NKJ, Fowler RA, Bhagwanjee S, Rubenfeld GD (2010) Critical care and the global burden of critical illness in adults. Lancet.
Alaparthi GK, Gatty A, Samuel SR, Amaravadi SK (2020) Effectiveness, safety, and barriers to early mobilisation in the intensive care unit. Crit Care Res Pract. 7840743.
Ali NA, O'Brien JM Jr, Hoffmann SP et al. (2008) Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 178(3):261-268.
Bailey P, Thomsen GE, Spuhler VJ et al. (2007) Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 35(1):139-45.
Baron MV, de Mello Pinto MV, Koepp J et al. (2019) Neuromuscular electrical stimulation in intensive care unit patients: Integrative review. Mod Res Inflamm. 8(2):11-27.
Burns JR, Jones FL Jr. (1975) Early ambulation of patients requiring ventilatory assistance. Chest. 68(4):608a.
De Jonghe B, Sharshar T, Lefaucheur JP et al. (2002) Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA. 288(22):2859-2867.
Dubb R, Nydahl P, Hermes C et al. (2016) Barriers and strategies for early mobilisation of patients in intensive care units. Ann Am Thorac Soc. 13(5):724-30.
Fossat G, Baudin F, Courtes L et al. (2018) Effect of in-bed leg cycling and electrical stimulation of the quadriceps on global muscle strength in critically ill adults: A randomized clinical trial. JAMA. 320(4):368-78.
Hermans G, Clerckx B, Vanhullebusch T et al. (2012) Interobserver agreement of medical research council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 45(1):18-25.
Hermans G, Van Mechelen H, Clerckx B et al. (2014) Acute outcomes and 1-year mortality of intensive care unit–acquired weakness: A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 190(4):410-420.
Kho ME, Damluji A, Zanni JM, Needham DM (2012) Feasibility and observed safety of interactive video games for physical rehabilitation in the intensive care unit: A case series. J Crit Care. 27(2):219.e1-6.
Kress JP, Hall JB (2014) ICU-acquired weakness and recovery from critical illness. N Engl J Med. 370(17):1626-1635.
Kress JP, Pohlman AS, O'Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 342(20):1471-1477.
Lordello GGG, Gama GGG, Ritt LEF (2020) Effects of cycle ergometer use in early mobilisation following cardiac surgery: A randomized controlled trial. Clin Rehabil. 34(4):485-94.
Mendez-Tellez PA, Dinglas VD, Colantuoni E et al. (2013) Factors associated with timing of initiation of physical therapy in acute lung injury patients. J Crit Care. 28(6):1061-6.
Moradian ST, Najafloo M, Mahmoudi H, Ghiasi MS (2017) Early mobilisation reduces the atelectasis and pleural effusion in patients undergoing coronary artery bypass graft surgery: A randomized clinical trial. J Vasc Nurs. 35(2):57-61.
Morris PE, Berry MJ, Files DC et al. (2016) Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: A randomized clinical trial. JAMA. 315(24):2694-702.
Moss M, Nordon-Craft A, Malone D et al. (2016) A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med. 193(10):1101-10.
Parry SM, Knight LD, Connolly B et al. (2017) Factors influencing physical activity and rehabilitation in survivors of critical illness: A systematic review of quantitative and qualitative studies. Intensive Care Med. 43(4):531-42.
Pun BT, Balas MC, Barnes-Daly MA et al. (2019) Caring for critically ill patients with the ABCDEF bundle: Results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 47(1):3-14.
Ries E (1899) Some radical changes in the after-treatment of celiotomy cases. JAMA. 33(8):454-456.
Santos PMR, Ricci NA, Suster ÉAB et al. (2016) Effects of early mobilisation in patients after cardiac surgery: A systematic review. Physiotherapy. 102(1):1-9.
Schaller SJ, Anstey M, Blobner M et al. (2016) Early, goal-directed mobilisation in the surgical intensive care unit: A randomised controlled trial. Lancet. 388(10052):1377-1388.
Schweickert WD, Pohlman MC, Pohlman AS et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 373(9678):1874-82.
The TEAM Study Investigators and the ANZICS Clinical Trials Group (2022) Early active mobilisation during mechanical ventilation in the ICU. N Engl J Med. 387(19):1747-58.
Thomsen GE, Snow GL, Rodriguez L, Hopkins RO (2008) Patients with respiratory failure increase ambulation when transferred to an intensive care unit where early mobility is a priority. Crit Care Med. 36(4):1119-24.
Tipping CJ, Bailey MJ, Bellomo R et al. (2016) The ICU Mobility Scale has construct and predictive validity and is responsive: A multicenter observational study. Ann Am Thorac Soc. 13(6):887-97.
Van Aerde N, Meersseman P, Debaveye Y et al. (2020) Five-year impact of ICU-acquired neuromuscular complications: A prospective, observational study. Intensive Care Med. 46(8):1184-1193.
Vasilevskis EE, Ely EW, Speroff T et al. (2010) Transparency in health care: Reducing iatrogenic risks—ICU-acquired delirium and weakness—Crossing the quality chasm. Chest. 138(5):1224-33.
Wolfe KS, Patel BK, MacKenzie EL et al. (2018) Kress JP. Impact of vasoactive medications on ICU-acquired weakness in mechanically ventilated patients. Chest. 154(4):781-787.
Zang K, Chen B, Wang M et al. (2019) The effect of early mobilisation in critically ill patients: A meta-analysis. Nurs Crit Care.
Zhang L, Hu W, Cai Z et al. (2019) Early mobilisation of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS One. 14(10):e0223185.
Zomorodi M, Topley D, McAnaw M (2012) Developing a mobility protocol for early mobilisation of patients in a surgical/trauma ICU. Crit Care Res Pract. 964547.








