ICU Management & Practice, ICU Volume 12 - Issue 1 - Spring 2012
Putting Evidence into Practice
Introduction
Central venous catheters are commonly used in intensive care units, with studies suggesting that approximately 50% of ICU patients have such lines inserted. Meanwhile, central line infections are responsible for 40-60% of bloodstream infections in intensive care patients, according to New South Wales Health (November 2008). These incidents are known as central line associated b - acteraemia (CLAB) and they significantly increase morbidity and mortality rates (Pittet et al. 1994; Soufir et al. 1999), as well as cost for the hospital system (Shannon et al. 2006).
A number of approaches have been used to decrease the risk of infection with central lines, with The Centres for Disease Control (CDC) 2002 guidelines (O'Grady N et al. 2002) emphasising the importance of aseptic insertion of central lines. Educational programmes encouraging this approach have successfully reduced CLAB rates (Coopersmith et al. 2002). A study by Berenholtz et al. (2004) demonstrated that CLAB rates could be dramatically reduced by using a quality improvement approach. Firstly, at Johns Hopkins Hospital, where rates fell from 11.3 to 0/1,000 line days, and then, in a large collaborative study in Michigan State. This involved 103 ICUs (85% of the ICU beds in that state) showing an 81% reduction in the mean rate of line days, which was estimated to have saved over 1,500 lives, 81,000 hospital days and 165 million US dollars (Pronovost et al. 2006). A recent Australian collaborative quality improvement project involving 37 ICUs also showed a reduction in CLAB rates from 3.0 to 1.2/1,000 line days (Burrell et al. 2011).
Given this evidence we decided to implement a multi-faceted quality improvement programme to reduce the CLAB rate at Middlemore Hospital’s Critical Care Complex, which in 2008 stood at 6.8/1,000 line days. Middlemore Hospital in Manukau City, New Zealand is an 800-bed facility that provides secondary and tertiary services to a population of 450,000. Throughout the programme, the seven beds provided in the ICU were increased to 12, with a six-bedded high dependency unit (HDU) added. This paper summarises the issues faced, what we did, our results, and the lessons learned.
Methods
We used the Institute for Healthcare Improvement’s CLAB prevention guide (2006) as the blueprint for our work, and used the CDC definition of a CLAB. In simple terms, a CLAB is defined as a significant bloodstream infection with no other obvious focus of infection, that occurs in a patient who either has a central line in place or had one applied within 48 hours of the blood cultures being taken (O'Grady et al. 2002).
Clinician Buy-In
Resistance to the programme from clinicians seemed to fall into four areas:
1. Denial that our rates were high and subsequent rejection that CLAB reduction was important;
2. Scepticism that a method used in the US would be applicable in New Zealand;
3. Unfamiliarity with a quality improvement approach, as opposed to the classic biomedical model. This challenged many of the clinicians who wanted large-scale trials, preferably randomised, on each and every aspect of the bundle; and
4. Resistance to standardisation of practice. We acknowledged that it was not useful to standardise most aspects of clinical decision-making, but that when the decision was made to insert a central line, it was useful to have a standardised approach to this. As Pronovost (2010) said, when placing a catheter, reliability not autonomy is needed.
Dixon-Woods et al. (2011) also identified resistance from doctors who were concerned that nurses would be monitoring their behaviour. The same study identified resistance from nurses who worried that doctors might react to being challenged. This was not an issue in our ICU, but these same sentiments were expressed in another department as we spread the programme throughout the hospital.
To build the will for change we presented the results from Michigan (Pronovost et al. 2006), and after many months of discussion, the Clinical Director of the Critical Care Complex made the decision to adopt the CLAB prevention bundle of care.
The CLAB prevention bundle was debated and then modified, with the team agreeing on four out of the five components:
1. Hand hygiene;
2. Chlorhexidine skin antisepsis (chlorhexidine 2% in 70% alcohol);
3.Maximum barrier precautions (hat, mask, sterile gloves, sterile gown and full patient drape); and
4. Daily review of the need for the line, with prompt removal of unnecessary lines.
The fifth component, using the subclavian as the preferred approach, was rejected by ICU staff, with evidence from Deshpande et al. (2005) supporting such decision. This modified bundle was incorporated into two checklists: one for line insertion, and one for daily maintenance of the lines (see Box 1). The layout of the checklists was modified over several months, following staff suggestions and investigation of CLAB cases (Seddon et al. 2011).
Keeping Momentum and Holding the Gains
We used visual feedback of results to build momentum. In a prominent place in the unit we displayed the:
• Days since the last CLAB. As we went on, CLABs became quite rare events and it was decided that the most useful way of showing this change was to display how many days there had been since our last CLAB. This was updated daily;
• Weekly compliance with the checklists. Initially this was a monthly figure, but this did not seem to have immediacy and also meant that we were too slow to detect drops in performance; and
• A six-month rolling rate of CLAB /1,000 line days.Although this rate smoothes the data and takes some months to show changes, it was the most useful visual feedback for medical clinicians (see Figure 1).
In the event of infection, each CLAB was defined as a sentinel occurrence and investigated. This reinforced for staff that CLABs were not just a part of everyday care for very sick patients.
Investigating these sentinel events also led to improvements as we were able to identify patients at high risk of CLAB: patients with severe immunosuppression, those receiving TPN, or those with large burns. For these patients a chlorhexidine biopatch was added, and antibioticimpregnated lines considered. Also at high risk were those that had lines inserted under emergency conditions, or in another hospital. These were flagged and lines were replaced at the earliest opportunity. The checklist was amended to check whether the patient fitted the high-risk criteria.
To further foster buy-in to the changes, we were keen to make the right thing to do the easiest thing to do, so we commissioned a central line insertion pack. This pack contained everything that was needed to insert a central line and was designed in such a way that it led the doctor through best practice. For example, the pack came with the insertion checklist, and on opening the pack, the first things encountered were the sterile hat and gloves. Compliance with the insertion and maintenance checklists was measured using an ‘all or none’ measure (Nolan and Berwick 2006), which specifies that all elements have to be accommodated.
Data from the laboratory (bloodstream infections) and the tally of central lines were used to determine two key measures:
• The CLAB rate per 1,000 line days; and
• The number of days between CLAB cases.
Results and Discussion
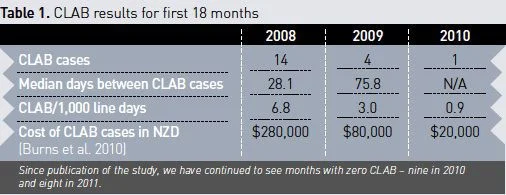
In 2008, before the CLAB initiative started, the ICU had 14 patients with CLAB, a median of 28.1 days (SD 2.1) between cases and a rate of 6.8/1,000 line days (see Table 1). Despite the increased bed numbers and workload in 2009, there were only four cases of CLAB, the median days between cases increased to 75.8 and the CLAB rate dropped to 3.0 CLAB/1,000 line days. In the first six months of 2010, the rate had dropped to 0.9 CLAB/1,000 line days.
With a multifaceted quality improvement programme and good clinical leadership, the team at Middlemore Hospital’s Critical Care Complex was able to reduce CLAB rates from 6.8 to 0.9/1,000 line days in 18 months.
Although we have undergone many months without a CLAB in the last year, we did have a cluster of five CLABs in a month and a half, so our rate over the last six months has risen accordingly (2.0/1,000 line days). The team did a full investigation of the five CLAB cases and found that most were high-risk patients but had not been recognised as such, and that line maintenance compliance had fallen from 80-90% to 40-50%. This was not apparent to staff at the time because we were only plotting monthly compliance rates. As a consequence, weekly rates were charted from then on, and senior charge nurses were asked to check compliance at the end of every shift. Following these changes, compliance improved and CLAB cases ceased. However, it should be pointed out that with any quality improvement programme, holding the gains requires vigilance.
We have found that this CLAB prevention work has had a positive effect on the patient safety culture of the unit. Staff are now working on a number of other initiatives, including ventilator-associated pneumonia, improved handovers and hand hygiene.