ICU Management & Practice, Volume 23 - Issue 4, 2023
Chronic obstructive pulmonary disease and asthma are pathologies par excellence with an obstructive pattern. It is important to understand the fundamentals of mechanical ventilation management in these patients. Therefore, it is crucial to perform proper measurement of respiratory mechanics in patients with obstructive pathology
Introduction and Justification
Chronic obstructive pulmonary disease (COPD) and asthma are pathologies par excellence with an obstructive pattern. According to a multicentre Spanish study assessing changes in the epidemiology of mechanical ventilation in Spain from 1998 to 2016, a significant shift in the reason for mechanical ventilation is observed, alongside a decrease in COPD from 12% to 5% as the cause for initiating mechanical ventilation. This decrease may be related to the increased use of non-invasive mechanical ventilation in this condition (Peñuelas et al. 2021). Regarding asthma, an American study estimates that out of the two million annual visits to the emergency department attributed to severe asthma exacerbations, approximately 25% of patients are hospitalised. Among these, between 5% and 10% require admission to the ICU, of which 2.1% require intubation and mechanical ventilation (Louie et al. 2012). As for the mortality of patients with obstructive respiratory pattern, it ranges from 11% to 32% in patients with COPD and from 1% to 7% in patients with asthma.
For all these reasons, it is important to understand the fundamentals of mechanical ventilation management in these patients (Gadre 2018).
Pathophysiology
In a mechanically ventilated patient, expiration occurs because the alveolar pressure (Palv) is greater than the airway pressure (Paw), producing a flow (Q) against an expiratory resistance (Raw) so that expiratory Q = (Palv - Paw)/ Raw. When airflow is obstructed, Raw increases, thus increasing the denominator and resulting in a decrease in expiratory Q.
It is believed that the mechanisms of airflow limitation are related to the collapsibility of the small airways. However, the anatomical location of this limitation is not entirely clear (Junhasavasdikul et al. 2018). In this situation of increased airway resistance and decreased expiratory flow, if we do not give sufficient expiratory time, there will be an increase in volume at the end of expiration beyond the functional residual capacity (FRC), resulting in hyperinflation and consequently in an end-expiratory pressure called intrinsic positive end-expiratory pressure (PEEPi). Therefore, early detection could help in the management of mechanically ventilated patients with flow limitation. This can be suspected using different means, but above all, by observing the ventilator curves.
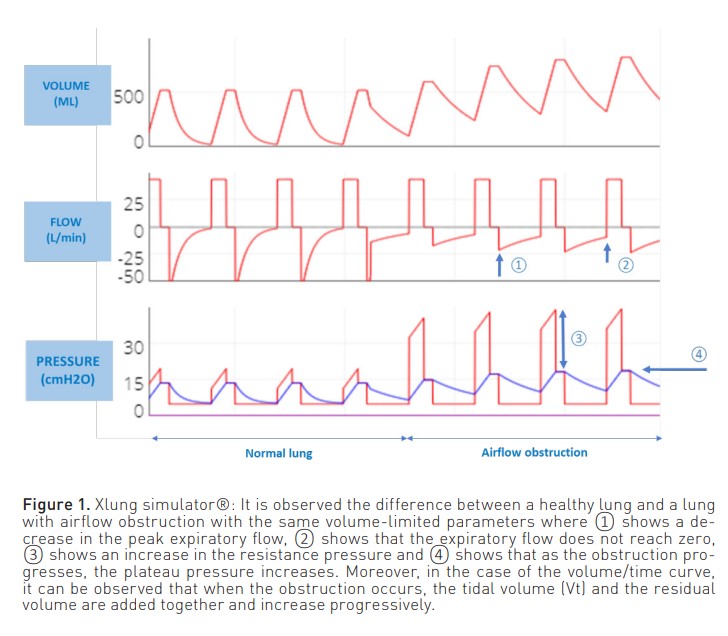
In patients ventilated in volume-limited mode (Figure 1), the inspiratory phase of the pressure curve shows an increase in airway resistance ((peak pressure - plateau pressure)/inspiratory flow). On the other hand, in the expiratory phase, there is a decrease in the peak expiratory flow. In addition, the expiratory curve does not reach zero because the respiratory system does not attain the FRC.
On the other hand, when the patient is ventilated in a pressure-limited mode (Figure 2), a reduction in both inspiratory and expiratory flow is observed, as well as a reduction in volume. Also, as with volume modes, the expiratory flow curve does not reach zero.
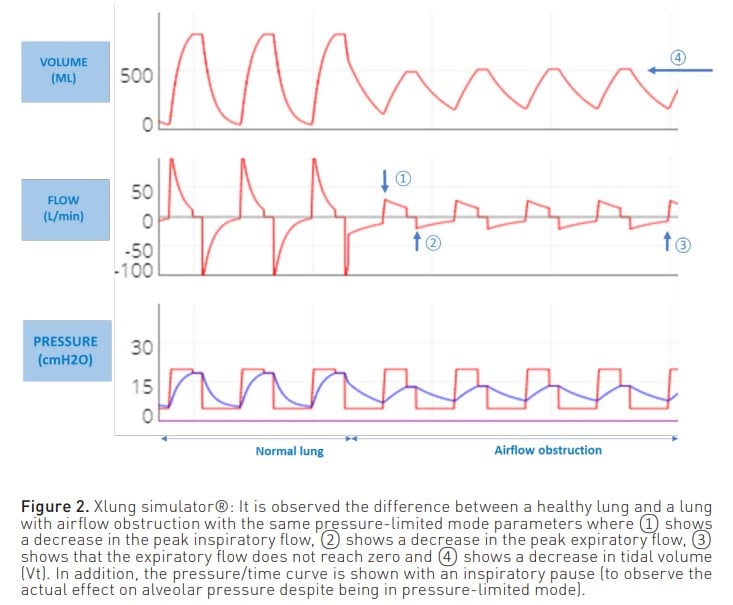
In both modes, this expiratory flow curve may show a sudden change in slope due to dynamic collapse and flow reduction, even reaching a square root morphology (Correger et al. 2012). In these patients, an increased peak pressure, an increased airway resistance pressure, a non-zero expiratory flow and, as entrapment progresses, an increase in plateau pressure can be observed.
Furthermore, it is essential to consider that entrapment can arise solely from improper ventilator programming, including the use of high respiratory rates that impede complete lung emptying. In a recent study, the presence of PEEPi and dynamic hyperinflation was evaluated in a large population of patients with acute respiratory distress syndrome (ARDS). The study concluded that in patients who are sedated and receiving neuromuscular relaxation without a known obstructive disease, PEEPi is insignificant and, therefore, does not affect respiratory mechanical properties (Coppola et al. 2019). Nonetheless, it is crucial to individualise each case. Accordingly, it is necessary to assess, in cases where shortening the expiratory time is required, the potential for air trapping and secondary effects on respiratory mechanics and haemodynamics, particularly when the patient is not heavily sedated or under neuromuscular relaxation.
Negative effects of hyperinflation
Hyperinflation has adverse effects:
- From a haemodynamic point of view, the increase in intrathoracic pressure due to air entrapment leads to a reduction in left ventricular end-diastolic volume and systolic volume with consequent arterial hypotension. If the haemodynamic consequences of PEEP are not recognised, it can lead to inadequate fluid restriction or unnecessary vasopressor treatment.
- On the other hand, at the pulmonary level, hypoventilation occurs despite an increase in minute volume. This is due to local overdistension of areas that do not empty on expiration, as well as the collapse of adjacent areas, thereby worsening ventilation. In cases where the pressure generated by hyperinflation is high, there is a risk of barotrauma and arterial hypotension. Therefore, whether due to its haemodynamic effects or its impact at the pulmonary level, arterial hypotension can be differentiated by a simple manoeuvre: disconnecting the patient from the ventilator for about 15 seconds. If the blood pressure rises after this manoeuvre, it is most likely that the cause of the hypotension is pulmonary hyperinflation. By disconnecting, the lungs have been emptied, and the hyperinflation has been reduced. If blood pressure does not rise after the manoeuvre, the possibility of pneumothorax must be considered.
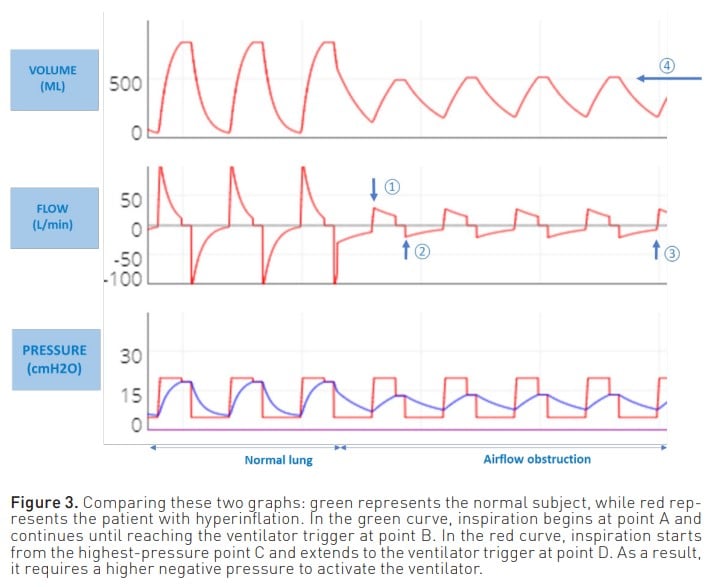
- Another adverse effect of air trapping is the increased work of breathing for the patient when initiating inspiration. This is because the patient must first overcome the pressure created by the trapped air to activate the ventilator. Consequently, greater effort and negative pressure are required to stimulate the trigger (Figure 3).
Measuring intrinsic PEEP
Given the effects of hyperinflation, it is important to measure PEEPi.
The most commonly used manoeuvre to measure PEEPi is to perform an expiratory pause of approximately 0.5 to 2 seconds. Thus, at the end of expiration both valves are closed, the pressures are equalised, and the pressure generated by the trapped air Figure 3. Comparing these two graphs: green represents the normal subject, while red represents the patient with hyperinflation. In the green curve, inspiration begins at point A and continues until reaching the ventilator trigger at point B. In the red curve, inspiration starts from the highest-pressure point C and extends to the ventilator trigger at point D. As a result, it requires a higher negative pressure to activate the ventilator. is measured. Although this is the most commonly used method for measuring PEEPi, it does not correlate with the risk of complications and is a poor estimator of true Palv.
The measurement that is associated with a higher risk of complications is end-inspiratory lung volume (Vei). This volume is the sum of the Vt and the trapped volume (Vee) (Figure 4). It is calculated by measuring the total volume of expired gas in a relaxed patient following a period of apnoea lasting 60 seconds, thereby allowing the lung to attain FRC.
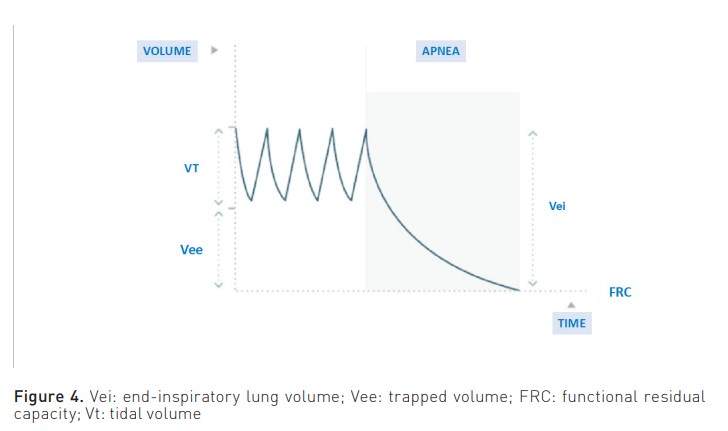
Therefore, a Vei surpassing 20 ml/kg is indicative of complications. The study by Roesthuis et al. (2021) examines bedside methods that offer easier implementation. These methods effectively capture the Vei, leading to the conclusion that airway pressures do not accurately represent the Vei in COPD patients. Furthermore, the three techniques employed to quantify Vei exhibit low bias but wide limits of agreement.
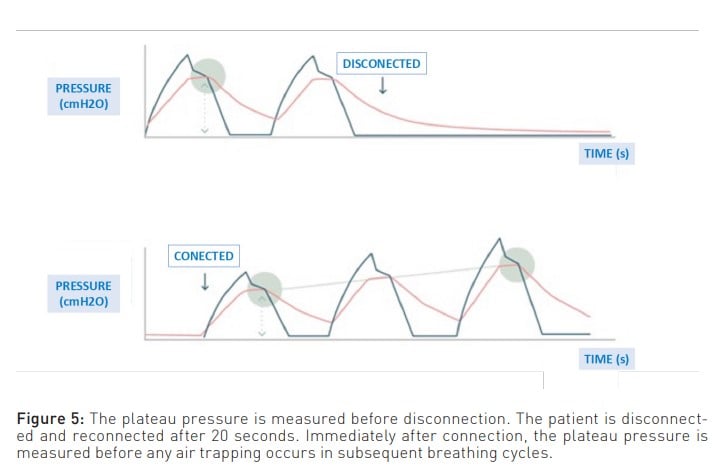
To enlarge upon the above, another limitation of measuring PEEPi using the expiratory pause manoeuvre is that it measures the air at the end of expiration. This air is in contact with the airway and, consequently, with the ventilator, which is where the pressure is measured. Therefore, it cannot measure the pressure generated by the air trapped behind the closed airways (Mannam 2010). This unmeasured pressure is known as ’hidden PEEP’. In this regard, one way to suspect that all the trapped air present is not measured is when, despite managing to decrease PEEPi through the various manoeuvres discussed below, the plateau pressure does not decrease. Moreover, a manoeuvre that provides a closer approximation to reality is the disconnect and reconnect method, where the difference in plateau pressure is measured before and after. This difference represents the pressure generated by the trapped air (Figure 5).
Management
The management of these patients is initially based on medical treatment and even non-invasive mechanical ventilation. However, close monitoring is of paramount importance because if there is no clinical improvement, endotracheal intubation and invasive mechanical ventilation should not be delayed.
The various manoeuvres to adjust ventilator parameters are aimed at increasing expiratory time (Garner et al. 2022). This can be accomplished by decreasing the respiratory rate and Vt, although these adjustments may result in hypoventilation and, subsequently, hypercapnia. Nevertheless, as long as it remains within the limits of what is known as permissive hypercapnia, which does not exceed 90 mmHg and maintains a pH above 7.15, it is considered acceptable and well-tolerated. Furthermore, other adjustments used to prolong expiratory time include increasing the inspiratory:expiratory ratio (I:E) without exceeding 4 seconds of expiratory time, as there is no evidence that more lung clearance is achieved beyond this time. Also, another approach is to increase the inspiratory flow without exceeding 50 cmH2 O of pressure because of this increase in flow. Finally, the last approach is to reduce the inspiratory pause if the patient is in a volume-limited mode.
Apart from this, no differences were found between pressure-limited and volumelimited ventilation modes in these patients. Therefore, the mode chosen is the one with which the specialist is more comfortable, but most importantly, the one that gives the best ventilation to the patient. In this regard, an advantage of the volume mode over the pressure mode is that the curves give more information, and more parameters can be adjusted to prolong the expiratory time.
The expiratory time constant (eTC) is a measure derived from the product of compliance and airway resistance (Demoule et al. 2020). The expiratory time is considered to be at least three times the eTC, so if it is less than twice the eTC, there is a risk of hyperinflation. However, in patients with obstructive pathology, the regional differences in mechanical properties preclude the use of a single eTC for the entire lung (Laghi et Goyal 2012).
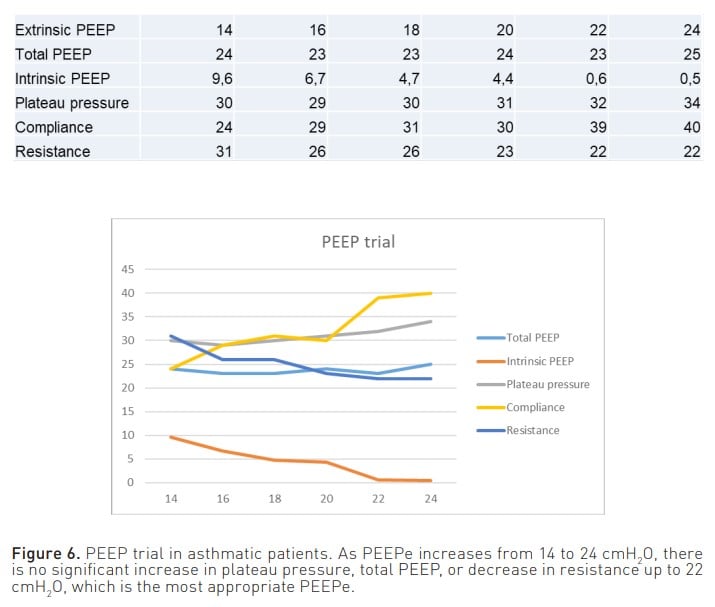
Furthermore, another important parameter to consider is the extrinsic PEEP (PEEPe). On the one hand, by appropriately adjusting this parameter, it is possible to achieve a decrease in one of the consequences of air entrapment, namely the workload experienced by the patient. When the pressure trigger is activated, with adequate PEEPe, the patient does not have to make an inspiratory effort from PEEPi to the trigger but from PEEPi to below PEEPe. On the other hand, PEEPe can also serve as a therapeutic measure, as it allows the opening of closed small airways and their emptying at the end of expiration. Moreover, it has been conventionally established that the PEEPe should be set at 80% of the PEEPi. However, considering that the manoeuvres used to measure PEEPi do not consider hidden PEEP, it is difficult to determine a percentage of pressure that cannot be reliably measured. Therefore, in a situation of air entrapment, to find the most appropriate PEEPe in each case to keep the airway open and achieve optimal lung emptying, it is recommended to perform a PEEP trial (Liang and Zhang 2016). This can be done by measuring plateau pressure and/or total PEEP (PEEPt) while increasing PEEPe by 2 cmH2 O per minute. When increasing the PEEPe, if there is no observed increase in plateau pressure or PEEPt, or if the increase is less than 2 cmH2 O, this would indicate lung emptying is taking place. On the contrary, once it is observed that the plateau pressure or PEEPt increases proportionally to the increase in PEEPe, it will be considered that there is no further lung emptying (Figure 6) (Abella et al. 2023). Therefore, the PEEPe that is prescribed is the one prior to the point at which lung emptying ceases.
Conclusion
The early recognition of air entrapment could aid in the management of mechanically ventilated patients with airflow obstruction. Moreover, this could potentially have an impact on the prognosis. Therefore, it is crucial to perform proper measurements of respiratory mechanics and consider the possibility of hidden PEEP.
To conclude, the objective of adjusting mechanical ventilation in obstructive pathology is to increase expiratory time and perform individualised PEEP trials for each case.
Conflict of Interest
None.