ICU Management & Practice, Volume 22 - Issue 5, 2022
Relevance
Plasma volume (PV) is the total volume of blood plasma – the extracellular fluid volume of the vascular space. It is associated with regulating interstitial and intravascular spaces; hence it can be an effective marker for volume overload (Kim et al. 2022).
Monitoring and managing volume status in critically ill patients are essential, whether in sepsis, cardiology, post-operatively or in dialysis (Metkus 2022; Rosner and Mullholland 2022). Traditionally, clinicians have relied on physical examination and physiologic variables such as heart rate and blood pressure to determine the need for fluid therapy. However, clinical examination alone is insufficient to guide this decision. Techniques that identify unstable patients and those who may respond to intravenous fluid are needed, as careful use of intravenous fluid is important for improved patient outcomes (Mackenzie and Noble 2014).
Estimated plasma volume (ePVS) is a useful diagnostic and prognostic tool. Elevated ePVS is associated with clinical outcomes in critically ill patients. ePVS has been found to be independently associated with cardiovascular outcomes, rehospitalisation, and death in patients with heart failure (HF) (Turcato et al. 2020). In patients with Acute Respiratory Distress Syndrome (ARDS), ePVS is associated with mortality and ICU- or ventilator-free days (Niedermeyer at al. 2021). A sustained increase in ePVS indicates a congestion status and is associated with a negative patient prognosis and increased mortality. Therefore, volume status is an equally relevant variable for therapeutic decision-making along with IV fluid administration, diuresis, treatment with vasopressors and intubation (Metkus 2022; Rosner and Mullholland 2022).
With ePVS determination and progress monitoring over time (ePV), volume status can be assessed. This allows for prompt initiation of therapy and, if necessary, an adjustment of therapy. In general, the measurement of PV is often difficult. Simple, non-invasive methods, such as medical history, weight, radiographs, and invasive techniques, such as transcardiopulmonary methods (PiCCO), are used for ePVS determination. Both approaches are labourious, costly, and not always available (Metkus 2022; Rosner and Mullholland 2022).
Alternatively, based on measured haemoglobin and haematocrit values, ePVS can be calculated using the Strauss formula:
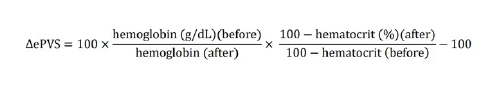
There is another formula that can also help with ePV estimation. It is an extension of the Strauss formula by Duarte et al. It provides an instantaneous measurement of PV using haematocrit and haemoglobin data from a single time-point (Kobayashi et al. 2021).

Clinical Studies and Case Studies
Congestion is a well-established predictor of outcomes in patients with HF, as it can lead to worsening disease and is associated with high mortality. In the event of inadequate therapy or residual congestion at discharge, there is a high risk of rehospitalisation. Therefore, a better understanding of the pathophysiology of congestion is extremely important, as is the need for finding more personalised therapies (Kobayashi et al. 2021; Boorsma et al. 2020).
In patients with acute HF, PV could increase by nearly 40%. This can lead to impairment of pulmonary function (Kobayashi et al. 2021). Volume overload with haemodynamic and clinical congestion can be a complex process in patients with acute and chronic HF. Multiple factors contribute to the accumulation and redistribution of fluid, ultimately resulting in volume overload and organ congestion. While clinical signs and symptoms can help alert clinicians of a change in volume status, there is still a need for quantitative measurement of blood volume in the patient as it can help guide treatment and/or adjust therapy (Miller 2017).
Findings from a study with 324 HF patients showed that the extent and composition of intravascular volume expansion significantly affected clinical outcomes. The impact of volume profiles varied with the progression of HF. Intravascular volume profiles were also predictive of the risk of HF admission, readmission or death (Kelly et al. 2021).
Transcatheter aortic valve implantation (TAVI) is an essential treatment option for severe aortic stenosis (AS). Subclinical congestion in patients undergoing TAVI is associated with worse clinical outcomes. However, this congestion often remains undetected during routine clinical assessment. Non-invasive techniques to calculate PV based on weight and haematocrit can improve prognosis in patients with HF. In 2021, in a prospective study of 859 patients undergoing TAVI, Seoudy et al. (2021) investigated the association between increased PV and poorer patient outcomes. Increased PV occurred in 535 patients. A significant increase in rehospitalisations and all-cause mortality within one year after TAVI (p=.001) were demonstrated. These findings show that increased PV in the subclinical range is a reliable marker (Seoudy et al. 2021).
In ARDS, a severe but common complication in ICU patients, optimal fluid management is extremely important (Niedermeyer at al. 2021).
In a study with 3165 ARDS patients a mean and median PVS of 5.9% was determined. Yet 68% of those patients had a positive PVS. Variations from the median were associated with outcome: a PVS above median resulted in a 30.6% mortality rate, whereas a lower PVS resulted in a 21.6% mortality rate (Niedermeyer et al. 2021).
Sepsis is often associated with haemorrhagic shock, Clarkson's syndrome and vasodilation. To ensure haemodynamic stability, plasma replacement therapy is often necessary (Marx et al. 2021). Volume status assessment and therapy monitoring are essential in these patients to detect and avoid lung or kidney congestion. Inadequate and aggressive fluid administration can lead to poor patient outcomes. Hence, fluid management needs to be carefully considered and monitored (Kalantari et al. 2013; Vincent 2019).
In a study with 1502 patients with fever at the emergency department, researchers evaluated the ePVS value registered at the time of admission and derived from complete blood count. 3.4% of the patients died at 30 days, and 5.3% of patients had a diagnosis of sepsis. The median ePVS in patients who died was higher compared to patients who survived (6.01dL/g vs 4.49dL/g, p<.0001). Hence, the ePVS value appears to be an effective tool for predicting the presence of sepsis and 30-day mortality (Turcato et al. 2020).
In another prospective study with 100 patients admitted to the ICU with sepsis or septic shock, in-hospital mortality was 47%, and the ePVS was found to be correlated with the amount of total fluids administered 24 hours before admission. The mean ePVS in patients who died was higher than in those who survived (7.7 ± 2.1 dL/g vs. 6.6 ± 1.6 dL/g, P = 0.003). These findings also show that ePVS can be used as a novel prognostic factor in patients with sepsis or septic shock.
Conclusion
The clinical evidence clearly shows the prognostic value of ePVS. Using Strauss or Duarte's formula to estimate PV is a useful strategy that can help improve patient outcomes. PV must be closely monitored and assessed through measurements of ePVS as ePVS is associated with in-hospital mortality and worsening outcomes. ePVS estimation remains an underutilised strategy despite clinical evidence of its prognostic value in heart failure and sepsis.
Key Points
- Monitoring and managing volume status in critically ill patients are essential, whether in sepsis, cardiology, post-operatively or in dialysis.
- Estimated plasma volume (ePVS) is a useful diagnostic and prognostic tool.
- Elevated ePVS is associated with clinical outcomes in critically ill patients.
- Volume overload with haemodynamic and clinical congestion can be a complex process in patients with acute and chronic HF.
- Volume status assessment and therapy monitoring are also essential in patients with sepsis.
- ePVS estimation remains an underutilised strategy despite clinical evidence of its prognostic value in critical care.
Disclaimer
Point-of-View articles are the sole opinion of the author(s) and they are part of the ICU Management & Practice Corporate Engagement or Educational Community Programme.
References:
Boorsma EM, Ter Maaten JM, Damman K et al. (2022) Congestion in heart
failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev
Cardiol. 17(10):641-655.
Kalantari K, Chang JN, Ronco C, Rosner MH (2013) Assessment of
intravascular volume status and volume responsiveness in critically ill
patients. Kidney Int. 83(6):1017-28.
Kelly KL, Wentz RJ, Johnson BD, Miller WL (2021) Relation of
Intravascular Volume Profiles to Heart Failure Progression and Clinical
Outcomes. Am J Cardiol. 153:65-70.
Kim KH, Cho HJ, Kim SC, Lee J (2022) Prognostic Value of Estimated
Plasma Volume Status in Patients With Sepsis. J Korean MedSci. 37(18):e145.
Kobayashi M, Girerd N, Duarte K et al. (2021) Estimated plasma volume status in heart failure: clinical implications and future directions. Clin Res Cardiol. 110(8):1159-1172.
Mackenzie DC, Noble VE (2014) Assessing volume status and fluid responsiveness in the emergency department. Clin Exp Emerg Med. 1(2):67-77.
Marx G, Zacharowski K, Ichai C et al. (2021) Efficacy and safety of
early target-controlled plasma volume replacement with a balanced gelatine
solution versus a balanced electrolyte solution in patients with severe
sepsis/septic shock: study protocol, design, and rationale of a prospective,
randomized, controlled, double-blind, multicentric, international clinical
trial: GENIUS-Gelatine use in ICU and sepsis. Trials. 22(1):376.
Metkus T (2022) Estimated Plasma Volume (ePV) in Critical Care. Nova Biomedical’s Educational Webinar Series. Available from novabiomedical.com/epv
Miller WL (2017)Assessment and Management of Volume Overload and
Congestion in Chronic Heart Failure: Can Measuring Blood Volume Provide New
Insights? Kidney Dis. 2(4):164-169.
Niedermeyer SE, Stephans RS, Kim BS, Metkus TS (2021) Calculated Plasma
Volume Is Associated With Mortality in Acute Respiratory Distress Syndrome.
Critical Care Explorations.3(9):e0534.
Rosner M, Mullholland H
(2022) Determining Intravascular Status: What Are My Options? Nova Biomedical’s
Educational Webinar Series. Available from novabiomedical.com/plasma-volume
Turcato G, Zaboli A, Ciccariello L, Pfeifer N (2020) Estimated plasma
volume status (ePVS) could be an easy-to-use clinical tool to determine the
risk of sepsis or death in patients with fever. J Crit Care. 58:106-112.
Vincent JL (2019) Fluid management in the critically ill. Kidney Int. 96(1):52-57.







