ICU Management & Practice, Volume 21 - Issue 1, 2021
Perioperative cardiorespiratory compromise is common and goes largely undetected. Predictive cardiorespiratory indices can help in early detection of harmful deviations and guide preemptive treatment. Using continuous cardiorespiratory monitoring coupled with these tools, we now know which patients are likely to decompensate both within and outside the operating room. There is a tremendous opportunity for artificial intelligence-based solutions to integrate these streams of continuous monitoring data with clinical records and design the next generation of quality and patient safety improvement measures.
Introduction
Despite major advances in perioperative care, postoperative complications are relatively common and a leading cause of death (Bartels et al. 2013). In fact the thirty days after non-cardiac surgery is still one of the leading causes of mortality in the United States (Carlberg et al. 2004). Perioperative physicians rely on continuous vital signs data to make important clinical decisions during a patient’s journey from the operating room, to the post-anaesthesia care unit, the general care floor and the intensive care unit. Physiologic monitoring data in combination with patient specific information in electronic health records can help build useful risk prediction tools. Not all patients may benefit from advanced continuous monitoring, and identifying high risk patient using predictive algorithms will allow patient specific recommendations. Also, clinical care information can help build and monitor quality metrics dashboards. The authors envision a not too distant future where these streams of data when used in context of natural language processing and free text from clinical notes, laboratory values and imaging studies, will develop dynamic prediction tools to improve perioperative patient safety. For example, perioperative intelligence (Maheshwari et al. 2019) can help advance perioperative medicine by focused application of artificial intelligence technologies in three key areas; identification of at-risk patients, early detection of complications, and timely and effective treatment.
Hypotension Prediction in the Perioperative Environment
Perioperative hypotension is strongly associated with myocardial injury after non-cardiac surgery (MINS) (Sessler et al. 2018). Identifying high risk patients can help target appropriate preventive and therapeutic measures. For example, routine screening for troponins in high risk patients can help detect MINS, in turn ensuring timely management including close monitoring, optimisation of haemodynamic status, and cardiology consultations as necessary (Devereaux and Sessler 2015). Patient baseline state, like age and comorbid conditions, can predict who will suffer from cardiorespiratory compromise. Revised Cardiac Risk Index (RCRI) (Lee et al. 1999) and American College of Surgeon’s National Surgical Quality Improvement Program (ACS NSQIP) (Bilimoria et al. 2013) are commonly used risk indices to calculate postoperative cardiac complications. When used in the right environment these risk prediction tools, can influence the decision for preoperative cardiac testing, surgical timing, and subsequent intraoperative and postoperative care.
Hypotension avoidance is a key to modern anaesthesia practice; however, not all hypotension can be avoided. Predicting hypotension in advance and instituting corrective actions proactively can help avoid it. For example, simple algorithms based on heart rate variability can help predict hypotension and bradycardia after induction of anaesthesia (Hanss et al. 2008). More complex machine learning based algorithm like Hypotension Prediction Index (HPI) (Hatib et al. 2018) which utilises arterial pressure waveform information can predict hypotension up to 15 minutes in advance. The HPI algorithm prediction performance seems optimal based on few retrospective and prospective studies (Davies et al. 2020; Maheshwari et al. 2020a) (Figure 1).
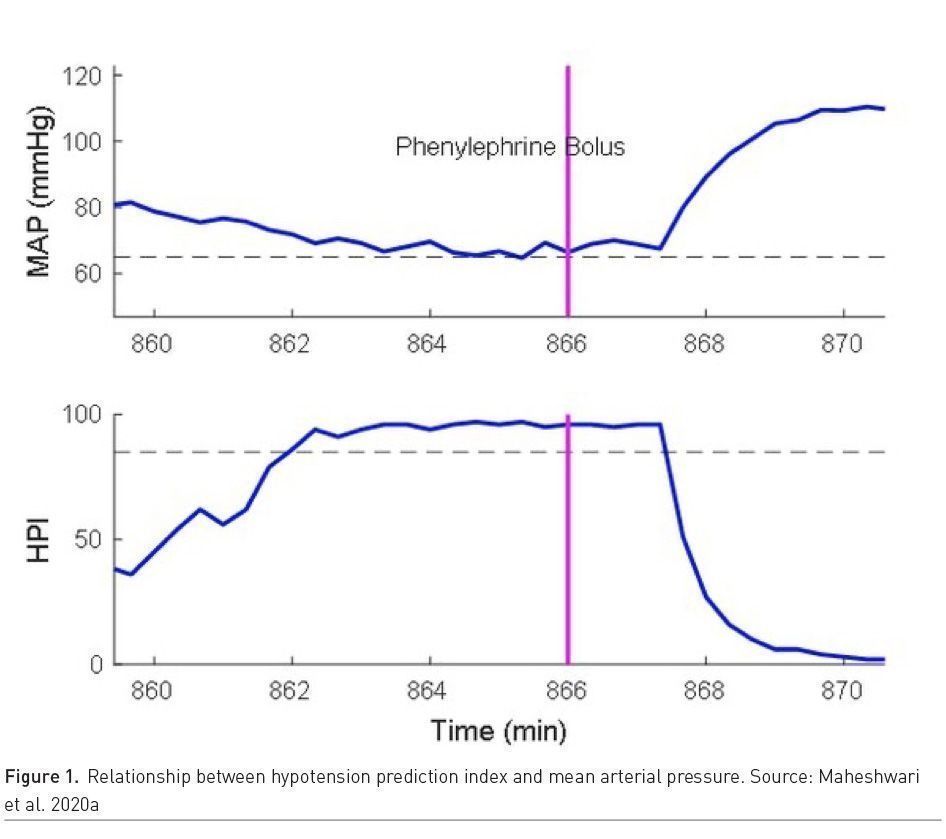
Two randomised trials have evaluated HPI performance in high risk patients. Winjeberge et al. (2020) randomised 60 patients undergoing moderate to high risk noncardiac surgery who required continuous invasive arterial pressure monitoring, half with HPI guidance and half without HPI guidance, and found 400% hypotension reduction with HPI guidance. Whereas, Maheshwari et al. (2020b), in a similar trial, randomised 214 patients and found no difference in hypotension among groups (http://youtu.be/2WGKrpiQA3s). Time between alert and hypotensive event, rate of baseline hypotension and treatment protocol compliance can explain some of the difference and needs to be studied further. In the critical care setting, hypotension is very common and prolonged, complicated in aetiology and pathogenesis, but hypotension prediction may help in timely treatment. For example, using Medical Information Mart for Intensive Care dataset (MIMIC III) database, Cherifa et al.(2020) used a Super Learner (SL) algorithm which is an ensemble machine-learning algorithm to predict an acute hypotensive episode 10 minutes in advance. Hypotension prediction in dynamic critical care setting is crucial for timely treatment. Algorithms which can help guide appropriate treatment and which result in better patient outcomes are needed. In addition, any hypotension prediction index in the ICU should be tested in a prospective trial and have an efferent arm (intervention arm) that is well defined.
Thus, evaluation of prediction system in real life clinical scenarios is critical. Not only do we need to confirm the prediction performance of a particular algorithm, that is, does it really predict a particular outcome, but equally important is how the intended use of an algorithm changes an outcome of interest in routine clinical care. The latter part is difficult and is currently lacking for most machine learning based algorithms used in perioperative care. We need to test these algorithms just like any other drug or device and show consistent outcomes improvement.
Respiratory Depression and Perioperative Pulmonary Complications Prediction
Postoperative respiratory complications are common (Miskovic and Lumb 2017). Alteration in respiratory drive, respiratory volumes and atelectasis can persist for up to six weeks after major surgery. Standardized Endpoints for Perioperative Medicine (StEP) Collaborative (Abbott et al. 2018) identified four important pulmonary outcomes; atelectasis detected on computed tomography or chest radiograph, pneumonia using U.S. Centers for Disease Control criteria, Acute Respiratory Distress Syndrome using Berlin consensus definition, pulmonary aspiration with clear clinical history and radiological evidence. We currently don’t have robust algorithms to predict or help prevent pulmonary complication and this will remain an area of research interest.
In addition, patients can have respiratory depression events and stories of tragic harm suffered by patients who arrested on the general care floor of the hospital (hospital ward code blue), are common. Sudden respiratory arrests are almost never truly sudden, and deterioration in vital signs happens for 4-6 hours before the index event (Saugel et al. 2020; Khanna et al. 2019). Slow deteriorations are usually missed because most monitoring on the hospital general care ward is intermittent every 4-6 hours, the world over (Weinger 2007). Large datasets with national resuscitation registries repeatedly show that unrecognised deterioration on the ward is common, and associated with persistent harm (Anderson et al. 2019). Prior work using continuous portable respiratory monitoring devices has shown that post-operative hypoxaemia is common, persistent and prolonged and goes undetected in up to 90% of patients (Sun et al. 2015). In addition, simple bedside tools such as the STOP-BANG score, or the type of opioids used do not effectively predict amount and duration of hypoxaemia (Khanna et al. 2016; Belcher et al. 2016). Hypoventilation and apnoea events are at least as common, if not more than hypoxaemic events (Khanna et al. 2020a; Overdyk et al. 2007). Portable, light-weight, accurate and well validated monitoring systems are needed not just for respiratory depression monitoring but also for assessment of continuous blood pressure on hospital ward patients (Saugel et al. 2020). One challenge is the burden of alarms that essentially convert a general hospital ward to a critical care unit, and the lack of effective intervention, that leads to increased burden for nursing staff and lack of proven benefit from large trials. Future technical advancements of ‘surveillance monitoring’ systems, will need an integrated EMR streaming, and an effective economic model that justifies large scale use across hospitals.
Currently, surveillance sensor products provide continuous data, with easy access to trends, and patterns, though blood pressure monitoring remains scarce. While universal continuous monitoring is an ambitious goal, we need to target monitoring and intervention to high risk patients. The PRODIGY is by far the largest prospective observational study of continuous, (alarms silenced and monitors blinded) oximetry and capnography, thus generating a sizeable data of vital sign parameters and corresponding outcome data in both surgical and medical patients (Khanna et al. 2020b). Using nearly 1500 patients across three continents over one year, continuous oximetry and capnography data was recorded in adults receiving opioids for pain management and nursing on the hospital general care ward. Monitors were covered and alarms silenced, while providers followed the usual, 4-6 hrs intervals vital signs checks. The recorded waveform data was analysed by an independent clinical event committee and artefacts were removed. Predictors of opioid induced respiratory depression were used in multivariate regression analysis to build a 5-factor risk prediction tool called the PRODIGY score (Table 1).
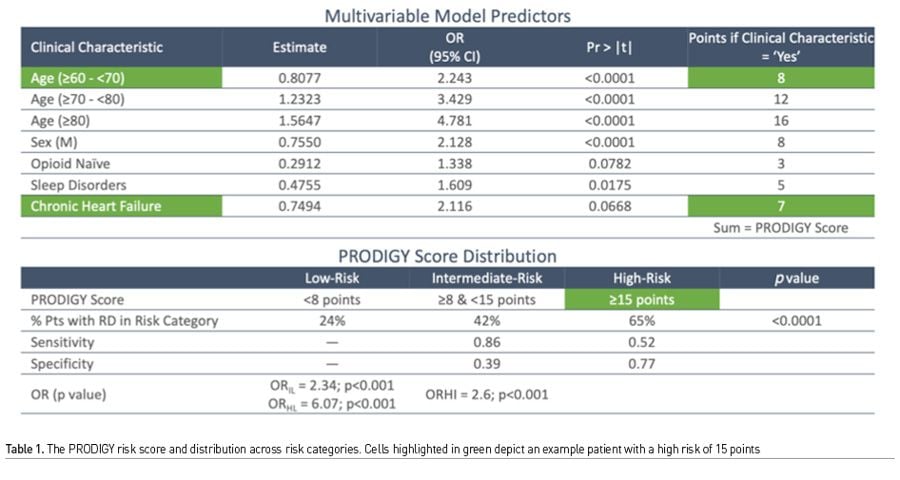
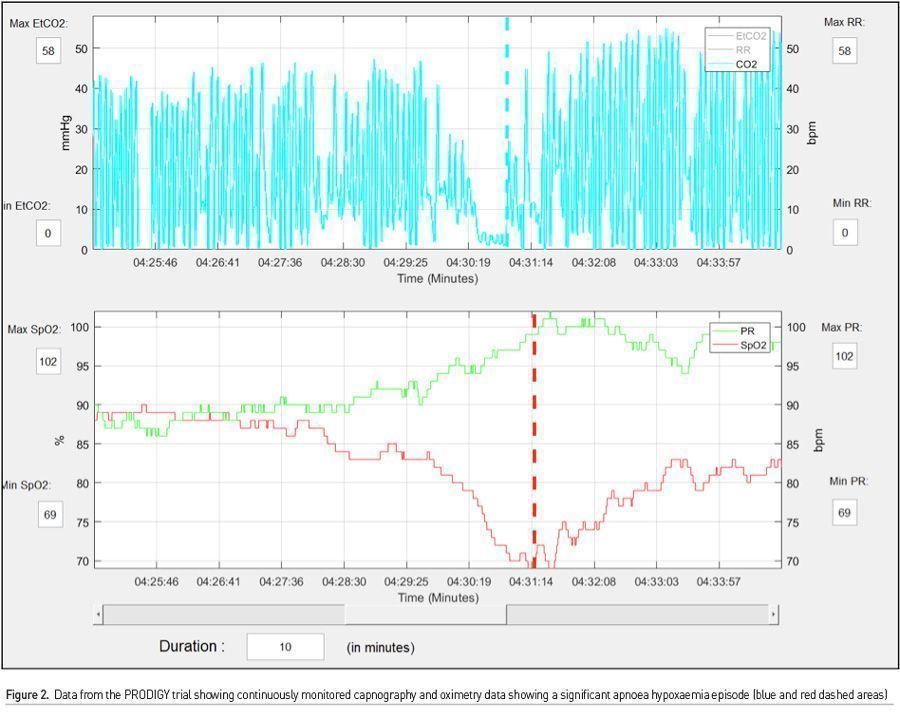
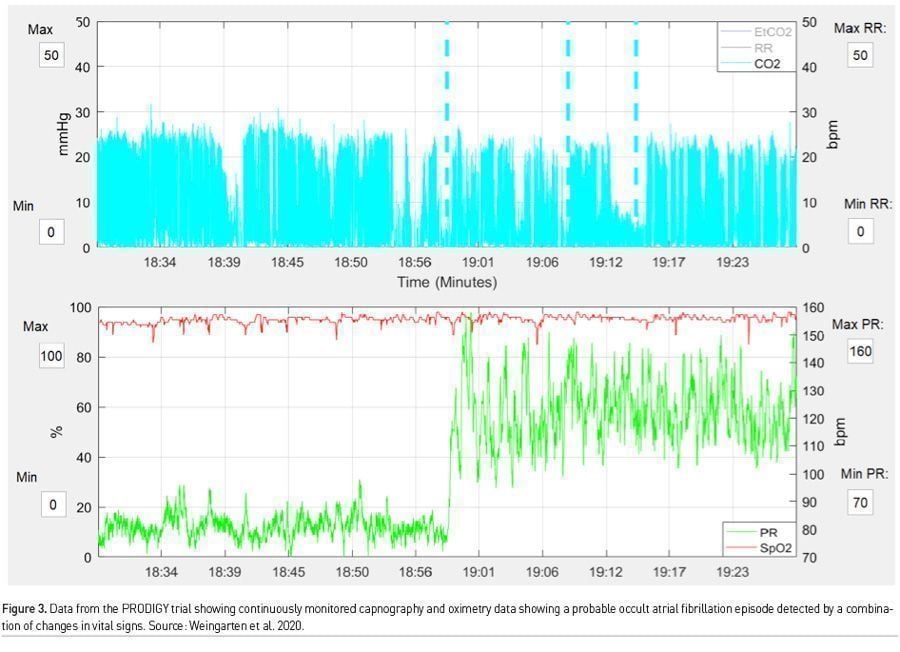
There is much to be learnt from the work surrounding PRODIGY. First, there were many more monitor detected respiratory depression episodes than we could ever imagine (46% of the total cohort), which would have gone unnoticed by traditional monitoring. Second, these episodes were not benign, and even though clinical events were few, patients with these episodes, had a mean length of hospital stay 3 days more than those who did not, and furthermore this translated into an exponential increase in hospital costs. Third, and most important, risk prediction using continuous monitoring offers the ability to trend oximetry and capnography data over time (Figures 2 & 3). Providing this data to clinicians would allow for pattern recognition and likely early intervention to correct cardiorespiratory compromise events. Artificial intelligence based learning and interpretation can provide critical insights, for example in sepsis detection (Yuan et al. 2020). Minimising false alarms is key for widespread clinical adoption.
Quality and Safety – Using AI to Build Connections
Machine learning (ML) and Artificial Intelligence (AI) have great potential to improve the quality and delivery of safe medical care in the perioperative period. Variability in clinical practice is one of the most significant drivers of perioperative complications (Lundberg et al. 2018). Universal adaptation of electronic medical records has allowed the acquisition of an enormous volume of clinical data; however, it put providers in a position of frequently not being able to process all available information in a timely and efficient manner. As such, important information may be missed delaying the right treatment or choosing the management plan, which is suboptimal. In the operating room, recovery room, or intensive care units, an inflow of patient data from monitors and clinical information available in medical records can be challenging to process and respond to. In particular, recognition of complex patterns in medical records along with continuous stream of monitoring data leading to undesirable outcome has been challenging to clinical providers. On the other hand, the advantage of ML and AI relies on their ability to sift through a massive amount of data, detect patterns and similarities, and provide decision support based on best and most successful scenarios learned from the analysis of similar cohorts in the past. Additionally, ML improves its predictive capabilties as more data becomes available and can update the models in real-time, making prediction or treatment suggestions clinically relevant and actionable. A comparison of population subsets based on outcomes further identifies the similarities and differences in patient characteristics, nuances of the procedures, and provided treatments. This insight can help provide compliance feedback with best practices to providers in near real-time when corrective intervention is still possible and potentially can change the clinical outcome trajectory. Avoidance of intraoperative hypoxia and arterial hypotension (described above) can be used as an example where ML and AI can significantly enhance providers' ability to deliver quality and safe patient care. Lundberg et al. developed an ML-based system to predict intraoperative hypoxia (Lundberg et al. 2018). The system was trained on minute-by-minute data from the electronic medical records of over fifty thousand surgeries. Providing anaesthesiologists with explainable hypoxaemia risks score and factors contributing to future hypoxia could allow intervention to modifiable risk factors before hypoxia occurred. Most importantly, the explanations identified by the algorithm for hypoxia predictions were broadly consistent with the literature and with prior knowledge from anaesthesiologists.
There are many examples of how AI and ML can help detect potentially harmful conditions during the perioperative period and help providers devise appropriate clinical responses. Wise implementation of these systems may improve the quality and safety of perioperative medical care. Instead of providing "reactive" intervention when cardiorespiratory complications already occur, AI may enable the ability to be "proactive" and avoid some of the complications altogether.
How Will AI Help in the Future?
Artificial intelligence is a computational science which attempts to replicate the complex task of information management similar to human brain. Machine learning, deep learning, natural language processing are various components of artificial intelligence which take data as input, process it through a learning model and provide a desirable output, commonly a prediction. Unlike prediction models or scores built on static retrospective data analysis, artificial intelligence gives us the ability to continuously collect data and improve these models without having to necessarily build new ones. Research and applications in various healthcare areas where cardiopulmonary deterioration needs to be monitored has been growing by leaps and bounds (Maheshwari et al. 2020b).
Data collection using various devices of the kinds described previously is the first step in developing any kind of intervention. Artificial intelligence holds the promise of detecting artefacts and outliers in the data while providing right data to right clinicians in real time for timely intervention (Ruskin and Hueske-Kraus 2015).
Development and implementation of AI models which can be deployed on monitoring devices themselves is a significant leap forward compared to retrospective data analysis. Complex indices or scores have been created using feature engineering, a process leveraged in AI to manage various variables and generate meaningful information. For example, scores such as hypotension prediction index, previously mentioned, in its development used millions of such pulse contour waveform features to predict hypotension (Hatib et al. 2018). In this regard, new scores such as the post-intubation hypotension prediction index and the post intubation hypoxaemia prediction index could also easily be built better with an AI model (Smischney et al. 2020a; Smischney et al. 2020b). The ability of AI to use multiple forms of data is also a unique characteristic which provides ability to not only use numerical data but also waveform data, text data amongst others.
Limitations of AI such as explainability, generalisability and bias for application across different clinical sites are getting addressed through newer and evolving techniques. Multi-site validation of various algorithms is also improving with more and more prospective randomised controlled studies being undertaken. With the recent advances in artificial intelligence including advancing research in healthcare, introduction of 5G telecommunication technology and application of internet of things (IOT) devices in healthcare, cardiopulmonary deterioration is likely to be detected and intervened upon earlier amongst perioperative patients.
Conclusion
The only way of preventing perioperative patient harm is continuous and better patient monitoring with an emphasis on early detection and prevention using effective therapeutic interventions. Tools such as HPI and PRODIGY need to be developed, and more importantly rigorously tested in appropriate clinical scenarios. Artificial intelligence applications to improve patient care and outcomes will need leadership, skilled personnel and right data handling infrastructure.
Conflict of Interest
AKK consults for Edwards Lifesciences, Medtronic, Philips North America, and Zoll Medical. AKK is on the clinical advisory board for Retia Medical and is a founding member for BrainX LLC, a collaborative platform for research and development of AI products in healthcare. PM is the founder of BrainXLLC, BrainX LLC and JC and KM are founding members. KM is a consultant for Edwards Lifesciences. AKK is also funded with a Clinical and Translational Science Institute (CTSI) NIH/NCTAS KL2 TR001421 award for a trial on continuous postoperative haemodynamic and saturation monitoring. 
References:
Abbott TEF, Fowler AJ, Pelosi P et al. (2018) A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth., 120(5):1066-1079.
Andersen LW, Holmberg MJ, Berg KM et al. (2019) In-Hospital Cardiac Arrest: A Review. JAMA, 321(12):1200-1210.
Bartels K, Karhausen J, Clambey ET et al. (2013) Perioperative organ injury. Anesthesiology, 119(6):1474-1489.
Belcher AW, Khanna AK, Leung S et al. (2016) Long-Acting Patient-Controlled Opioids Are Not Associated With More Postoperative Hypoxemia Than Short-Acting Patient-Controlled Opioids After Noncardiac Surgery: A Cohort Analysis. Anesth Analg., 123(6):1471-1479.
Bilimoria KY, Liu Y, Paruch JL et al. (2013) Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg., 217(5):833-842 e831-833.
Carlberg B, Samuelsson O, Lindholm LH (2004) Atenolol in hypertension: is it a wise choice? Lancet, 364(9446):1684-1689.
Cherifa M, Blet A, Chambaz A et al. (2020) Prediction of an Acute Hypotensive Episode During an ICU Hospitalization With a Super Learner Machine-Learning Algorithm. Anesth Analg., 130(5):1157-1166.
Davies SJ, Vistisen ST, Jian Z et al. (2020) Ability of an Arterial Waveform Analysis-Derived Hypotension Prediction Index to Predict Future Hypotensive Events in Surgical Patients. Anesth Analg., 130(2):352-359.
Devereaux PJ, Sessler DI (2015) Cardiac Complications in Patients Undergoing Major Noncardiac Surgery. N Engl J Med.,373(23):2258-2269.
Hanss R, Renner J, Ilies C et al. (2008) Does heart rate variability predict hypotension and bradycardia after induction of general anaesthesia in high risk cardiovascular patients? Anaesthesia, 63(2):129-135.
Hatib F, Jian Z, Buddi S et al. (2018) Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology, 129(4):663-674.
Khanna AK, Hoppe P, Saugel B (2019) Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care, 23(1):194.
Khanna AK, Sessler DI, Sun Z, et al. (2016) Using the STOP-BANG questionnaire to predict hypoxaemia in patients recovering from noncardiac surgery: a prospective cohort analysis. Br J Anaesth., 116(5):632-640.
Khanna AK, Bergese SD, Jungquist CR et al. (2020a) Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg., 131(4):1012-1024.
Khanna AK, Bergese SD, Jungquist CR, et al. (2020b) Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg., Publish Ahead of Print.
Lee TH, Marcantonio ER, Mangione CM et al. (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation, 100(10):1043-1049.
Lundberg SM, Nair B, Vavilala MS et al. (2018) Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat Biomed Eng., 2(10):749-760.
Maheshwari K, Ruetzler K, Saugel B (2019) Perioperative intelligence: applications of artificial intelligence in perioperative medicine. J Clin Monit Comput.
Maheshwari K, Buddi S, Jian Z et al. (2020a) Performance of the Hypotension Prediction Index with non-invasive arterial pressure waveforms in non-cardiac surgical patients. Journal of Clinical Monitoring and Computing.
Maheshwari K, Shimada T, Yang D, et al. (2020b) Hypotension Prediction Index for Prevention of Hypotension during Moderate- to High-risk Noncardiac Surgery. Anesthesiology.
Miskovic A, Lumb AB (2017) Postoperative pulmonary complications. Br J Anaesth., 118(3):317-334.
Overdyk FJ, Carter R, Maddox RR et al. (2007) Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg., 105(2):412-418.
Ruskin KJ, Hueske-Kraus D (2015) Alarm fatigue: impacts on patient safety. Curr Opin Anaesthesiol., 28(6):685-690.
Saugel B, Hoppe P, Khanna AK (2020) Automated Continuous Noninvasive Ward Monitoring: Validation of Measurement Systems Is the Real Challenge. Anesthesiology, 132(3):407-410.
Sessler DI, Meyhoff CS, Zimmerman NM et al. (2018) Period-dependent Associations between Hypotension during and for Four Days after Noncardiac Surgery and a Composite of Myocardial Infarction and Death: A Substudy of the POISE-2 Trial. Anesthesiology, 128(2):317-327.
Smischney NJ, Khanna AK, Brauer E et al. (2020a) Risk Factors for and Outcomes Associated With Peri-Intubation Hypoxemia: A Multicenter Prospective Cohort Study. J Intensive Care Med. 2020:885066620962445.
Smischney NJ, Kashyap R, Khanna AK et al. (2020b) Risk factors for and prediction of post-intubation hypotension in critically ill adults: A multicenter prospective cohort study. PLoS One. 2020;15(8):e0233852.
Sun Z, Sessler DI, Dalton JE et al. (2015) Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth Analg., 121(3):709-715.
Weingarten TN, Morimatsu H, Fiorda-Diaz J et al. (2020) New-Onset Atrial Fibrillation Detected by Continuous Capnography Monitoring: A Case Report. The American Journal of Case Reports, 21:e925510-1
Weinger MB (2007) Dangers of postoperative opioids. APSF Newsletter, 21(4):61-67.
Wijnberge M, Geerts BF, Hol L et al. (2020) Effect of a Machine Learning-Derived Early Warning System for Intraoperative Hypotension vs Standard Care on Depth and Duration of Intraoperative Hypotension During Elective Noncardiac Surgery: The HYPE Randomized Clinical Trial. JAMA.
Yuan KC, Tsai LW, Lee KH, et al. (2020) The development an artificial intelligence algorithm for early sepsis diagnosis in the intensive care unit. International journal of medical informatics, 141:104176.










