The role of nutrition in obese critically ill patients and an overview of the clinical guidelines for nutrition provision in this patient population.
Introduction
The World Health Organization defines obesity as an excess of abdominal fat that poses an increased risk to health. Characterised by a body mass index (BMI) ≥30 kg/m2, obesity rates have tripled since 1975, and in 2016, 650 million people worldwide were obese (who.int/news-room/fact-sheets/detail/obesity-and-overweight). In the largest analysis of international nutrition provision during critical illness (n=17,154), more than half of the patients were overweight or obese, and the mean and standard deviation (SD) BMI was 27 (8) kg/m2 (Ridley et al. 2018). Moreover, in the most recently published and largest critical care enteral nutrition trial ever conducted, the impact of higher energy enteral feeding versus standard care nutrition on 90-day survival was investigated (3957 patients from 46 ICUs in Australia and New Zealand). The mean (SD) BMI in the intervention and standard care groups was 29.2 (7.7) kg/m2 and 29.3 (7.9) kg/m2 respectively (Chapman et al. 2018). Obesity is associated with increased morbidity in the general population, but the impact of obesity in critical illness on clinical outcomes is more complex. While obesity is associated with increased morbidity and resource utilisation, a J-shaped relationship exists where overweight and moderate obesity is protective of mortality compared to a normal BMI or severe obesity [known as the obesity paradox] (Arroyo-Johnson and Mincey 2016; Schetz et al. 2019).
Clinical Guidelines Informing Nutrition Provision in Obese Critically Ill Adults
Published first, The American Society of Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines: Nutrition Support of Hospitalized Adult Patients inform the ASPEN/Society of Critical Care Medicine Guidelines for the Provision and Assessment of Nutrition Support Therapy. Both of these guidelines recommend hypocaloric energy provision (lower than measured or estimated energy expenditure) with high protein intake for hospitalised and critically ill obese patients based on 2 available RCTs and limited observational evidence (Choban et al. 2013; McClave et al. 2016). Hypocaloric energy provision is recommended as obese hospitalised patients are at increased risk of metabolic complications if overfeeding occurs (Choban et al. 2013). The basis for the higher protein recommendations is to modulate catabolism and facilitate protein anabolism. The amount of protein recommended increases with class of obesity and are based on data from 163 patients in total (Table 1) (Choban et al. 2013; McClave et al. 2016). It must be noted that the recommendations are being extrapolated to critically ill obese patients when only some of the data have been derived in this population, and positive nitrogen balance and protein anabolism is very difficult to achieve in critical illness due to catabolic metabolic processes, especially in the early phase of illness. However, it is entirely plausible that protein may be more important than energy in critical illness, and this may vary depending on phase of illness; however, as with the general critically ill population, definitive data is required to understand this. In contrast, the most recent European Society of Parenteral and Enteral Nutrition (ESPEN) guidelines on clinical nutrition in the ICU recommend isocaloric energy intake with 1.3 g/kg of protein using an adjusted body weight (Singer et al. 2018). Table 1 summarises the ASPEN (McClave et al. 2016) and ESPEN (Singer et al. 2018) clinical guideline recommendations for the nutrition management of critically ill obese patients.

Evidence Informing Nutrition Provision in Critically Ill Obese Adults
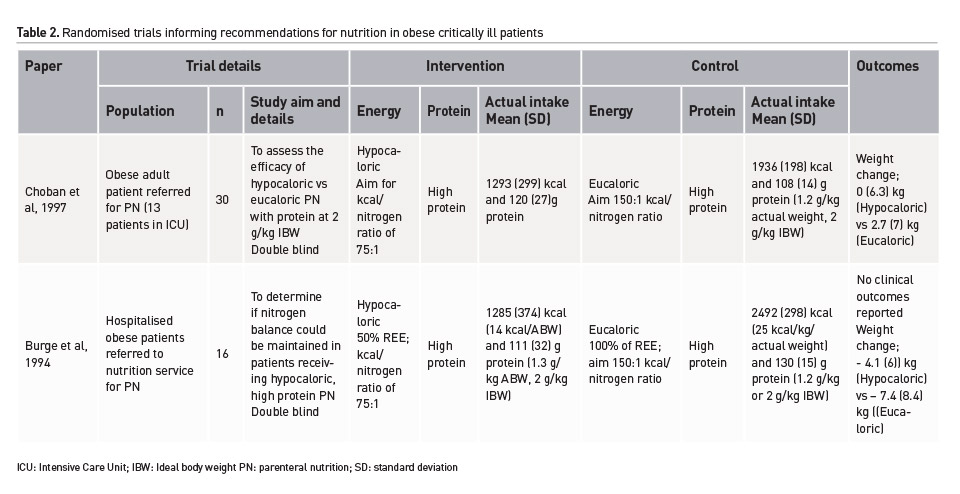
Minimal high-quality research exists investigating the impact of nutrition on clinical and functional outcomes in critically ill obese patients. Two, double-blind, randomised controlled trials (RCTs) have been conducted over 20 years ago. Including less than 50 patients in total, both investigated hypocaloric, high protein PN interventions, only one was conducted in a critically ill population, and both were clearly underpowered to investigate important clinical outcomes (Burge et al. 1994; Choban et al. 1997). Table 2 provides a summary of these trials. Conversely, the largest observational analysis available (162 critically ill patients with a BMI of 35-40 kg/m2 out of a total sample of 2772) found a significant survival association with additional energy and protein above standard care (Alberda et al. 2009). This finding can only be considered hypothesis generating despite statistical adjustment and analysis due to the considerable risk of confounding. Furthermore the previously mentioned enteral feeding trial (The Augmented Versus Routine Approach to Giving Energy Trial), enrolled the largest cohort of critically ill obese patients within a robust RCT design (n=1423 with a BMI ≥ 30 kg/m2). The treatment effect on 90-day survival was not statistically significant although the obese sub-group was the only group where the point-estimate favoured the intervention of higher energy delivery (Chapman et al. 2018). Importantly, patients in both groups received the same amount of protein (1.1g/kg ideal body weight/day).
Considerations for Clinicians
For clinicians to fully understand the impact of nutrition in obese critically ill patients, several fundamental issues need to considered and robustly investigated. Firstly, commonly used predictive equations are less accurate in overweight and obese patients compared to those of normal weight. This is probably due to the most commonly used equations being developed in non-obese populations but applied in those with obesity, coupled with the considerable variation in body composition in individuals who are obese (Frankfield et al. 2005; Frankfield et al. 2013). For example, an obese person can carry a high muscle mass, be very physically active, and be metabolically healthy, or they can suffer from malnutrition and sarcopenic obesity. This variation in body composition is also why the use of BMI as a ‘marker’ of obesity is sub-optimal as it does not consider the distribution of muscle and adipose tissue (Choban et al. 2013). However, the assessment of muscularity is challenging in the ICU setting, and obesity adds another element of difficulty with excessive adipose tissue making a physical assessment almost impossible (Sheean et al. 2014). Currently, methods to objectively measure or predict whole-body muscle in critically ill patients are limited (Earthman 2015). CT image analysis at the third lumbar area can be used, although this technology needs specialist training and is clearly limited to a select group of patients who have a CT scan at L3 (Paris and Mourtzakis 2016; Price and Earthman 2019).
Ultrasonography and bioimpedance analysis show promise and studies are underway to investigate these methods further, although use in the obese population may be limited (clinicaltrials.gov/show/NCT03019913). Variations in body composition can also cause significant differences in metabolic rate (high in those with increased muscle mass and low in those with sarcopenia) and the response to nutrition delivery may hence be varied. Clinicians should, therefore, consider that metabolic rate is likely to be variable in obese critically ill patients. In a recent cohort study of 25 critically ill patients with a BMI ≥ 30 kg/m2, the mean measured resting metabolic rate (RMR) using indirect calorimetry was 2506 (749) kcal. The predicted energy requirement using the ASPEN guidelines recommendation of 11-14 kcal/kg/actual body weight was 1080 (200) and 1375 (254) kcal/day, respectively (Vest et al. 2019). This is an alarming difference when there are no definitive data on the clinical and functional consequences of hypocaloric feeding strategies in obese critically ill patients. In contrast, a recent RCT investigating the use of indirect calorimetry (intervention) to guide nutrition delivery compared to a predictive estimate (standard care) included patients with a median BMI of approximately 22 kg/m2. In this population who were largely in the healthy weight range, the median RMR (interquartile range) was 2069 (1816–2380) kcal in the intervention group and 1887 (1674–2244) kcal in the standard care (Allingstrup et al. 2017). It is therefore plausible that in some obese patients, energy expenditure may be higher than predicted by equations (especially in the acute flow phase of illness). Given the differences observed between measured estimates and the ASPEN hypocaloric nutrition guidelines, it is hypothesised that a minimum weight loss of 2-3 kg per week could be induced if nutrition were prescribed according to these guidelines (Singer et al. 2018). Furthermore, clinicians should be aware that when aiming for full target nutrition during critical illness, patients almost always receive approximately 50-60% of this goal for multifactorial reasons (Ridley et al. 2018; Passier et al. 2013). It is likely that if hypocaloric nutrition is purposefully aimed for, even less will be achieved, without understanding the clinical and functional impact. Finally, a large observational analysis of 3257 ICU stays investigated the association of BMI with the timing of the commencement of nutrition support. A BMI ≥30 kg/m2 (n=663/3257) was independently associated with a longer time to initiation of nutrition than any other BMI category (relative risk for delayed nutrition commencement in obese patients; 1.06 (1.00, 1.12) for obese patients, P = 0.004) (Borel et al. 2014). The reason for this was not examined, but it could be hypothesised that it reflects an assumption that commencement of nutrition in obese critically ill patients is not prioritised as it is in those of normal or low body weight.
Clinical Implications for Clinicians
It is the opinion of the authors that until definitive research is achieved as to the impact of energy and protein delivery on clinical and quality of life outcomes, critically ill obese adults, be managed as any other critically ill patient. Evidence from a number of large RCTs suggests that the amount of energy delivered during the first week of ICU has no impact on survival or functional outcomes (Needham et al. 2013). Given the inaccuracy of predictive equations, indirect calorimetry is preferred to calculate energy expenditure. If predictive equations are used, an adjusted weight should be calculated to account for excess adiposity for both the energy and protein estimations. Consideration to body composition and pre-morbid function should also be given and may inform expected energy expenditure (high or low). Enteral administration of some nutrition should be commenced as early as possible during the ICU stay and increased to goal as tolerated. Inability to deliver full energy goals in the first week of the ICU stay should not result in the initiation of extraordinary treatments (such as the administration of prokinetics, the placement of small intestinal feeding tubes or the intravenous administration of nutrition) as these treatments may have adverse effects and no benefit on outcome has been demonstrated early in ICU stay. After the first week of ICU stay, 80-100% of energy and protein goals should be achieved based on the possibility that significant weight loss during a catabolic period may lead to the development of sarcopenia with persistent obesity, compromising functional recovery. As recommended in ESPEN guidelines, a protein intake of at least 1.3 g/kg adjusted body weight delivered should be the aim until definitive evidence is achieved as to the impact of higher protein delivery on clinical and functional outcomes (Singer et al. 2018). Moreover, achieving higher protein delivery is difficult with current commercially available products, and without definitive evidence seems unnecessary. It may be appropriate to consider a weight loss regime once transitioned to the ward; however, this should be assessed on an individual basis with a multidisciplinary team.
Conclusion
The need for a robustly designed and systematic programme of research to investigate the role of nutrition in obese critically ill patients has been recommended since 2002 and most recently in an important clinical guideline; however no RCTs have been performed, and there are none registered on any major trial registries (Choban et al. 2013; Dickerson et al. 2002). Well-designed and adequately powered studies are now urgently needed to understand the energy and protein requirement to target, the impact of energy and protein delivery, and to address the important question of whether a hypocaloric, high protein diet improves important clinical and functional outcomes in obese critically ill adults. Until such time it is recommended that clinicians manage the nutrition of the obese critically ill patient as any other patient; conservatively in the first week of ICU stay, with an aim to meet energy and protein requirements after this time, recognising that metabolic rate may be highly variable based on body composition, and prolonged starvation may impact functional recovery.
Key Points
- Obesity is associated with increased morbidity in the general population, but the impact of obesity in critical illness on clinical outcomes is more complex.
- Clinical guidelines recommend hypocaloric energy provision with high protein intake for hospitalised and critically ill obese patients.
- Commonly used predictive equations are less accurate in overweight and obese patients compared to those of normal weight, and indirect calorimetry is preferred to calculate energy expenditure.
- Clinicians should manage the nutrition of the obese critically ill patient as any other patient; conservatively in the first week of ICU stay, with an aim to meet energy and protein requirements after this time.
Alberda
C, Gramlich L, Jones N et al. (2009) The relationship between nutritional
intake and clinical
outcomes in critically ill patients: results of an international multicenter
observational study.
Intensive Care Med, 35(10):1728-37.
Allingstrup
MJ, Kondrup J, Wiis J et al. (2017) Early goal-directed nutrition versus
standard of care
in adult intensive care patients: the single-centre, randomised, outcome
assessor-blinded
EAT-ICU trial. Intensive Care Med, 43(11):1637-47.
Arroyo-Johnson C, Mincey KD (2016) Obesity Epidemiology Worldwide.
Gastroenterol Clin North Am,
45(4):571-9.
Borel
AL, Schwebel C, Planquette B et al. (2014) Initiation of nutritional support is
delayed in
critically ill obese patients: a multicenter cohort study. Am J Clin Nutr, 100(3):859-66.
Burge
JC, Goon A, Choban PS et al. (1994) Efficacy of hypocaloric total parenteral
nutrition in
hospitalized obese patients: a prospective, double-blind randomized trial. JPEN
J Parenter Enteral
Nutr, 18(3):203-7.
Chapman
M, Peake SL, Target Investigators et al. (2018) Energy-Dense versus Routine Enteral
Nutrition in the Critically Ill. New England Journal of Medicine, 379(19):1823-34.
Choban
P, Dickerson R, Malone A et al. (2013) A.S.P.E.N. Clinical guidelines:
nutrition support
of hospitalized adult patients with
obesity. JPEN J Parenter Enteral Nutr, 37(6):714-44.
Choban PS, Burge JC, Scales D et al. (1997) Hypoenergetic
nutrition support in hospitalized obese patients: a simplified method for
clinical application. Am J Clin Nutr 1997, 66(3):546-50.
Dickerson RN, Boschert KJ, Kudsk KA et al. (2002) Hypocaloric
enteral tube feeding in critically ill obese patients. Nutrition 2002,
18(3):241-6
Earthman
CP (2015) Body Composition Tools for Assessment of Adult Malnutrition at the Bedside:
A Tutorial on Research Considerations and Clinical Applications. JPEN J
Parenter Enteral
Nutr, 39(7):787-822.
Evaluation
of Bedside Methods to Measure Muscularity in Critically Ill Patients. Available from clinicaltrials.gov/show/NCT03019913
Frankenfield D, Roth-Yousey L, Compher C (2005) Comparison of
predictive equations for resting metabolic rate in healthy nonobese and obese
adults: a systematic review. J Am Diet Assoc, 105(5):775-89.
Frankenfield
DC, Ashcraft CM, Galvan DA (2013) Prediction of resting metabolic rate in critically
ill patients at the extremes of body mass index. JPEN J Parenter Enteral Nutr 2013;37(3):361-7.
McClave SA, Taylor BE, Martindale RG et al. (2016) Guidelines for the Provision and Assessment
of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical
Care Medicine (SCCM) and American
Society for Parenteral and Enteral Nutrition (A.S.P.E.N.).
JPEN J Parenter Enteral Nutr, 40(2):159-211.
Needham
DM, Dinglas VD, Morris PE et al. (2013) Physical and cognitive performance of patients
with acute lung injury 1 year after
initial trophic versus full enteral feeding. EDEN trial
follow-up. Am J Respir Crit Care Med, 188(5):567-76.
Overweight and obesity (2018) World
Health Organization. Available from who.int/newsroom/fact-sheets/detail/obesity-and-overweight
Paris
M, Mourtzakis M (2016) Assessment of skeletal muscle mass in critically ill
patients: considerations
for the utility of computed tomography imaging and ultrasonography. Curr Opin
Clin Nutr Metab Care, 19(2):125-30.
Passier
RH, Davies AR, Ridley E et al. (2013) Periprocedural cessation of nutrition in
the intensive
care unit: opportunities for improvement. Intensive Care Med, 39(7):1221-6.
Price KL, Earthman CP (2019) Update on body composition tools
in clinical settings: computed tomography, ultrasound, and bioimpedance
applications for assessment and
monitoring.
Eur J Clin Nutr, 73(2):187-93.
Ridley EJ, Peake SL, Jarvis M et al. (2018) Nutrition Therapy
in Australia and New Zealand Intensive Care Units: An International Comparison
Study. JPEN J Parenter Enteral Nutr, 42(8):1349-57.
Schetz M, De Jong A, Deane AM, et al. (2019) Obesity in the
critically ill: a narrative review. Intensive Care Med, 45(6):757-69.
Sheean
PM, Peterson SJ, Gomez Perez S et al. (2014) The prevalence of sarcopenia in patients
with respiratory failure classified as normally nourished using computed tomography
and
subjective global assessment. JPEN J Parenter Enteral Nutr, 38(7):873-9.
Singer P, Blaser AR, Berger MM et al. (2018) ESPEN guideline
on clinical nutrition in the intensive care unit. Clin Nutr.
Vest MT, Newell E, Shapero M et al. (2019) Energy balance in
obese, mechanically ventilated
intensive care unit patients. Nutrition, 66:48-53.












