ICU Management & Practice, Volume 22 - Issue 3, 2022
The prevalence of obesity is increasing worldwide (Schetz et al. 2019). This trend is confirmed in the Intensive Care Unit (ICU) where patients with obesity represent 15% to 40% of the population (Schetz et al. 2019; De Jong et al. 2018b; De Jong et al. 2019; De Jong et al. 2018a). Moreover, obesity has several implications for critical illness due to the difficulties of caring for such patients, including positioning, transport, skin care, intravascular access, diagnostic imaging, and ventilator weaning (De Jong et al. 2020; Pepin et al. 2016).
Obesity exerts physical, metabolic, and molecular effects across multiple organ systems and is associated with numerous comorbidities (diabetes, cardiovascular diseases, hypertension, chronic kidney disease, dyslipidaemia, non-alcoholic fatty liver disease, obstructive sleep apnoea and hypoventilation syndrome, mood disorders and physical disabilities) (Schetz et al. 2019). This underlying pathophysiological setting has both direct and indirect impacts in cri tically ill patients with obesity (Schetz et al. 2019; Barletta and Erstad 2022; Plečko 2021).
Pain in the ICU
In addition to the treatment of various organ failures in the ICU, one of the challenges is pain management (Kalfon et al. 2020; de Jong et al. 2013). Pain assessment in the critically ill adult remains a daily clinical challenge. Indeed, pain has been shown to be experienced at rest by more than 30% of patients (Chanques et al. 2007) and this percentage exceeds 50% during common care procedures in ICU (Puntillo et al. 2014).
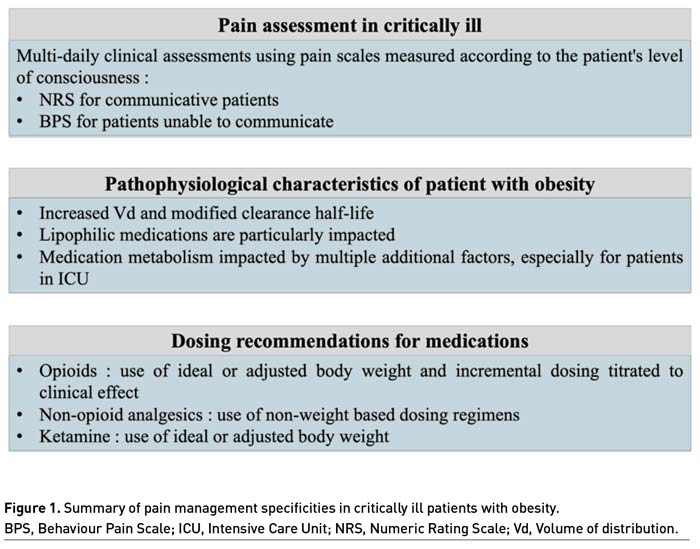
Pain should be monitored routinely in all adult intensive care patients, using validated scales according to the patient's level of consciousness (Devlin et al. 2018; Chanques 2022). Briefly, the 0-10 Numeric Rating Scale (NRS) is commonly used in clinical practice, and an enlarged visual format of the NRS was found to be the most feasible and discriminative self-report scale in comparison to other scales (i.e., visual analogue scale, verbal descriptor scale) and formats (i.e., oral versus visual) for measuring pain intensity in critically ill adult patients (Chanques et al. 2010).
Behaviour Pain Scale (BPS) is used as the gold standard to measure pain in the population of ICU patients unable to communicate according to guidelines, especially in the sedated and mechanically ventilated patient (Chanques et al. 2020; Chanques et al. 2014; Devlin et al. 2018). The BPS is composed of three criteria: facial expression, upper limbs and compliance with ventilation. Each parameter is scored from 1 to 4 by trained staff and results in a score between 3 and 12 (Payen et al. 2001). In non-intubated ICU patients unable to communicate, pain level can be assessed with the BPS-NI scale (Chanques et al. 2009).
Depending on the numerical value obtained using these pain assessment scales, it is possible to classify pain as moderate or severe on a daily basis and to monitor its evolution during the stay (de Jong et al. 2013).
Pain and Obesity
Obesity is associated with chronic pain by several mechanisms including a mechanical impairment from excessive weight on skeletal muscles and joints and an altered systematic inflammatory status (Janke et al. 2007). However, cause-and-effect relationships between obesity and pain is not clear and cannot be extrapolated to the phenomenon of acute pain. The current literature provides contradictory results, especially in pain sensitivity of patients with obesity (Torensma et al. 2017).
Recent experimental studies support an increase in sensory pain thresholds through several mechanisms. The first hypothesis is mechanical with the evidence of higher thresholds and lower subjective ratings in patients with obesity, especially in areas with excess subcutaneous fat (Torensma et al. 2017; Price et al. 2013). This could be explained by the stretching of the skin due to excess fat, which leads to a decrease in the density of the nerve fibres, and therefore the pain thresholds.
The second hypothesis relates to an endocrine pattern with an inflammatory environment that could also increase sensory pain thresholds. Excess adipose tissue in patients with obesity is highly metabolically active, and especially visceral adipose tissue which has a deleterious adipocyte secretory profile resulting in insulin resistance and a chronic low-grade inflammatory and procoagulant state (Piché et al. 2018; Neeland et al. 2018).
At the hormonal level, difference in concentrations between patients with and without obesity has been demonstrated for galanin (Yu et al. 2013) and b-endorphin (Price et al. 2013) with higher sensory pain thresholds in patients with obesity.
The last hypothesis still under study is a central dysregulation, including altered regulation of the neurovegetative system (Chanques et al. 2017) associated with neuropsychological changes in patients with obesity (Torensma et al. 2017).
Pharmacokinetics and Pharmacodynamics in Patients With Obesity
Obesity can affect pharmacokinetics (relationship between drug dose and concentrations in the body) as well as pharmacodynamics (the pharmacologic effect resulting from a drug’s concentration). Recommendations for medication dosing in critically ill obese patients are not available from adequately powered randomised studies with clinically relevant endpoints.
Medication dosing regimens are often determined by cohorts of normal weight participants, raising questions about their applicability to patients with obesity in whom clearance and volume of distribution (Vd) may be substantially different. Weight-based dosing guidelines often do not specify the use of Actual Body Weight (ABW) versus ideal (based on height and sex) or adjusted (typically between actual and ideal) weight estimates (Barletta and Erstad 2022) and multiple additional factors impacted by obesity must be considered for appropriate dosing. Further, equations to estimate lean body mass are not reliable in critically ill patients when compared to computed tomography as the gold standard (Moisey et al. 2017).
Multiple additional factors influenced by obesity must be considered for appropriate dosing, such as the presence of diabetes that can lead to glomerular hyperfiltration, or hepatic steatosis that may decrease the clearance of hepatically metabolised medications. In addition, association with one or more organ dysfunctions (e.g., acute kidney injury), may complicate dose selection.
Vd, calculated by dividing the total amount of drug in the body by the plasma concentration, is influenced by medication lipophilicity, molecular size, and protein binding, which alter a drug’s ability to move between blood and tissues. Therefore, lipophilic medications may have a larger Vd in patients with obesity, requiring higher loading doses (Barletta and Erstad 2022). Furthermore, these medications have increased elimination half-life explained by multicompartmental pharmacokinetic model with redistribution phenomena before elimination.
Therapeutic Adjustments
Erstad and Barletta (2020) recently proposed a review of the literature based on drug dosing in ICU to develop recommendations for patient with severe obesity (i.e. BMI ≥ 40 kg/m2) in the areas of analgesia, sedation and delirium. Three major therapies were studied for analgesia: opioids, non-opioid analgesics, and ketamine.
In studies suggesting a size descriptor for dosing opioids, recommendations were for ideal body weight, lean body mass, or adjusted body weight as a preferred descriptor, because prospective and retrospective studies performed in the emergency department and post-operative setting have consistently found large variations in opioid requirements and pain control in overweight and patients with obesity that had no relationship to ABW (Xia et al. 2014; Bennett et al. 1982). Similarly, pharmacokinetic studies evaluating various opioids in the perioperative setting have found opioid doses based on ABW are likely to be excessive as evidenced by pharmacokinetic parameters and measured opioid concentrations (Egan et al. 1998; Slepchenko et al. 2003). For dosing opioids, incremental dosing titrated to clinical effect with consistent use of an ideal or adjusted body weight is suggested for weight-based dosing particularly in patients with more severe forms of obesity, to reduce the risks related to overdosing.
Non-opioid analgesics, such as nonsteroidal anti-inflammatory drugs and acetaminophen commonly administered to critically ill patients, typically use non-weight-based dosing regimens. The few pharmacokinetic and pharmacodynamic studies evaluating the disposition of non-opioid agents show little benefit for dose individualisation based on weight with adverse effect (especially liver disease) when increasing doses beyond those needed to reach the analgesic ceiling effect (Motov et al. 2017; Allard et al. 2019). For dosing non-opioids analgesics, non-weight based dosing regimens are advised.
Regarding ketamine, this molecule has substantial lipophilicity with a large Vd, rapid clearance and active metabolites, all complicate potential dosing recommendations (Clements and Nimmo 1981) and especially in ICU (Hijazi et al. 2003). To loading doses, ABW is appealing as a size descriptor given the lipophilicity of ketamine, since clinical effect in this situation is largely a function of the drug’s Vd. With sustained intermittent intravenous injections or continuous infusions of ketamine, accumulation of both parent drug and active metabolite norketamine occurs until steady state conditions occur. Norketamine has one-third the potency of the parent compound, but also has slower elimination that increases the time to reach steady state, thus probably requiring a decrease in dose over time to maintain the same clinical effect. In consequence, the complex estimation of clearance of ketamine (due to a lack of correlation between lean body mass and fat mass in patients with obesity) combined with the complicated determination of an active metabolite suggests the use of ideal or adjusted body weight is preferable for weight-based dosing calculations due to adverse effect concerns associated with overdosing.
Conclusion
In the current state of knowledge and in the face of the complex interrelationships among pain, body weight, comorbid conditions and behavioural/biological contributors, the pain management in patients with obesity in ICU has only recently been described. Further studies are needed to develop analgesia protocols specifically designed for patients with obesity.
In addition, the management of pain in patients with obesity requires, as for any other patients in ICU, multi-daily clinical assessments using pain scales measured according to the patient's level of consciousness to assess pain evolution.
For patients with obesity, there is no high-level clinical evidence available to help design dosing regimens for analgesia in critically ill.
Based on pharmacokinetic studies, the relationship between ABW and pharmacokinetic variables such as Vd and clearance is not linear for most of pain medications. For such medications, standard, non-weight-based dosing, or weight-based dosing using either ideal body weight or adjusted body weight, is appropriate to limit the risk of overdosing. In patients with obesity as in all patients who received a sustained infusion of sedatives and/or opioids, repeated assessment of clinical needs (sedation level, pain intensity) are mandatory to titrate the dose and to avoid analgo-sedation side-effects related to its overuse.
Conflict of Interest
Audrey De Jong reports receiving consulting fees from Medtronic and Drager. Gerald Chanques and Ambre Cuny have no conflict of interest.
References:
Allard J, Le Guillou D, Begriche K, Fromenty B (2019) Drug-induced liver injury in obesity and nonalcoholic fatty liver disease. Adv Pharmacol. 85:75-107.
Barletta JF, Erstrad BL (2022) Drug dosing in hospitalized obese patients with COVID-19. Crit Care 26, 60
Bennett R, Batenhorst R, Graves DA et al. (1982) Variation in postoperative analgesic requirements in the morbidly obese following gastric bypass surgery. Pharmacotherapy. 2:50-3.
Chanques G (2022) Pain assessment in critical illness. ICU Management and Practice. 22(3):106-111.
Chanques G, Constantin JM, Devlin JW et al. (2020) Analgesia and sedation in patients with ARDS. Intensive Care Med. 46:2342-2356.
Chanques G, Payen JF, Mercier G et al. (2009) Assessing pain in non-intubated critically ill patients unable to self report: an adaptation of the Behavioral Pain Scale. Intensive Care Med. 35: 2060-7.
Chanques G, Pohlman A, Kress JP et al. (2014) Psychometric comparison of three behavioural
scales for the assessment of pain in critically ill patients unable to self-report. Crit Care. 18:R160.
Chanques G, Sebbane M, Barbotte E et al. (2007) A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology. 107:858-60.
Chanques G, Tarri T, Ride A et al. (2017) Analgesia nociception index for the assessment of pain in critically ill patients: a diagnostic accuracy study. Br J Anaesth. 119:812-820.
Chanques G, Viel E, Constantin JM et al. (2010) The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain. 151:711-21.
Clements JA, Nimmo WS (1981) Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 53:27-30.
De Jong A, Cossic J, Verzilli D et al. (2018a) Impact of the driving pressure on mortality in obese and non-obese ARDS patients: a retrospective study of 362 cases. Intensive Care Med. 44:1106-1114.
De Jong A, Molinari N, De Lattre S et al. (2013) Decreasing severe pain and serious adverse events while moving intensive care unit patients: a prospective interventional study (the NURSE-DO project). Crit Care. 17: R74.
De Jong A, Verzilli D, Jaber S (2019) ARDS in Obese Patients: Specificities and Management. Crit Care. 23, 74.
De Jong A, Verzilli D, Sebbane M et al. (2018b) Medical Versus Surgical ICU Obese Patient Outcome: A Propensity-Matched Analysis to Resolve Clinical Trial Controversies. Crit Care Med. 46:e294-e301.
De Jong A, Wrigge H, Hedenstierna G et al. (2020) How to ventilate obese patients in the ICU. Intensive Care Med. 46:2423-2435.
Devlin JW, Skrobik Y, Gélinas, C et al. (2018) Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 46: e825-e873.
Egan TD, Huizinga B, Gupta SK et al. (1998) Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology. 89:562-73.
Erstad BL, Barletta JF (2020) Drug dosing in the critically ill obese patient-a focus on sedation, analgesia, and delirium. Crit Care. 24, 315.
Hijazi Y, Bodonian C, Bolon M et al. (2003) Pharmacokinetics and haemodynamics of ketaminein intensive care patients with brain or spinal cord injury. Br J Anaesth. 90:155-60.
Janke EA, Collins A, Kozak AT (2007) Overview of the relationship between pain and obesity: What do we know? Where do we go next? J Rehabil Res Dev. 44:245-62.
Kalfon P, Boucekine M, Estagnasie P et al. (2020) Risk factors and events in the adult intensive care unit associated with pain as self-reported at the end of the intensive care unit stay. Crit Care. 24, 685.
Moisey LL, Mourtzakis M, Kozar RA et al. (2017) Existing equations to estimate lean body mass are not accurate in the critically ill: Results of a multicenter observational study. Clin Nutr. 36:1701-1706.
Motov S, Yasavolian M, Likourezos A et al. (2017) Comparison of Intravenous Ketorolac at Three Single-Dose Regimens for Treating Acute Pain in the Emergency Department: A Randomized Controlled Trial. Ann Emerg Med. 70:177-184.
Neeland IJ, Poirier P, Després JP (2018) Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 137:1391-1406.
Payen JF, Bru O, Bosson JL et al. (2001) Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 29:2258-63.
Pepin JL, Timsit JF, Tamisier R et al. (2016) Prevention and care of respiratory failure in obese patients. Lancet Respir Med. 4:407-18.
Piché ME, Poirier P, Lemieux I, Després JP (2018) Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Prog Cardiovasc Dis. 61:103-113.
Plečko D, Bennett N, Mårtensson J, Bellomo R (2021) The obesity paradox and hypoglycemia in critically ill patients. Crit Care. 25, 378.
Price RC, Asenjo JF, Christou NV et al. (2013) The role of excess subcutaneous fat in pain and sensory sensitivity in obesity. Eur J Pain. 17:1316-26.
Puntillo KA, Max A, Timsit JF et al. (2014) Determinants of procedural pain intensity in the intensive care unit. The Europain(R) study. Am J Respir Crit Care Med. 189:39-47.
Schetz M, De Jong A, Deane AM et al. (2019) Obesity in the critically ill: a narrative review. Intensive Care Med. 45:757-769.
Slepchenko G, Simon N, Goubaux B et al. (2003) Performance of target-controlled sufentanil infusion in obese patients. Anesthesiology. 98:65-73.
Torensma B, Oudejans L, Van Velzen M et al. (2017) Pain sensitivity and pain scoring in patients with morbid obesity. Surg Obes Relat Dis. 13:788-795.
Xia S, Choe D, Hernandez L, Birnbaum A (2014) Does initial hydromorphone relieve pain best if dosing is fixed or weight based? Ann Emerg Med. 63: 692-8.e4.
Yu M, Fang P, Shi M et al. (2013) Galanin receptors possibly modulate the obesity-induced change in pain threshold. Peptides. 44:55-9.









