ICU Management & Practice, Volume 19 - Issue 1, 2019
Finding patients before they crash might be the next major opportunity to
improve patient safety. This article describes recent advances and perspectives
for ward monitoring with wearable sensors and smart algorithms.
Hospital wards are dangerous. Indeed, they are where most unexpected deaths occur within institutions. In a UK national audit study, among 23,554 adult in-hospital cardiac arrests, more than half (57%) occurred on the wards and only 5% in the ICU (Nolan et al. 2014). In the large (>46,000 patients) EUSOS study done in 28 European countries, most patients (73%) who died before hospital discharge were not admitted to critical care at any stage after surgery (Pearse et al. 2012). Importantly, most patients do not suddenly deteriorate. Vital signs are often abnormal, or trending toward abnormal range, hours before cardiac arrest or ICU transfer (Churpek et al. 2012). But healthcare professionals may only suddenly notice this is happening because spot-checks are usually done on a 4-6 hours interval. Prospective observational studies conducted in a leading U.S hospital, where patients were continuously but blindly monitored, revealed that nurses who were checking vital signs every 4h missed 90% of hypoxaemic episodes and about half of hypotensive events (Sun et al. 2015; Turan et al. 2019).
Therefore, the introduction of continuous monitoring has the potential to improve quality of care in traditionally unmonitored settings (Abenstein 2010; Michard and Sessler 2018; Vincent et al. 2018). With the current rise of wearable products developed for health and fitness (e.g. smartwatches detecting arrhythmia or devices monitoring pulse oxygen saturation (SpO2) and blood pressure (BP) when connected to a smartphone) we are about to reach an unprecedented situation: monitoring may become more intensive at home than in hospital wards so that staying at home may paradoxically be considered safer.
In this article, we summarise which physiologic variables should be monitored, which tools are currently available to do so, and discuss requirements for the future development and adoption of continuous monitoring solutions as standard of care for hospital wards.
You might also like: Innovations in monitoring: from smartphones to wearables
Which variables should we monitor?
Vital signs classically spot-checked in ward patients include heart rate (HR), BP, respiratory rate (RR), SpO2 and temperature. They all have potential value to detect clinical deterioration. Some are more sensitive than specific, like HR that may increase during many situations, including postoperative pain, circulatory shock, respiratory distress, and sepsis. Others are more specific than sensitive. For instance, SpO2 would decrease only in case of respiratory failure, pending oxygen administration is not automatically titrated to maintain a normal SpO2 (L’Her et al. 2017). SpO2 has also been considered a lagging indicator in acute events of respiratory depression.
The ability of vital signs to predict clinical deterioration depends on the clinical context. During patient-controlled analgesia with opioids, rates of desaturation (SpO2< 90%) and bradypnoea (RR < 10) lasting >3 min can reach 12% and 41%, respectively (Overdyk et al. 2007). In this context, monitoring respiratory variables, in particular, RR, becomes a priority. In contrast, monitoring temperature and HR would likely be more useful to detect sepsis and prompt appropriate biological samples and treatments. A recent nationwide study (Michard et al. 2015) including >200,000 patients from >500 US hospitals showed that most common postoperative adverse events are respiratory and infectious complications, emphasising the importance of monitoring RR, SpO2, HR and temperature.
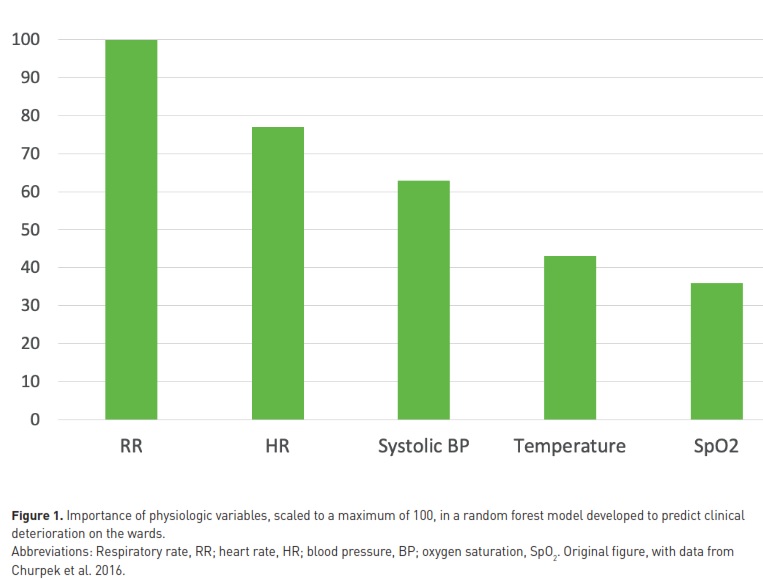
In the general medical and surgical ward population, studies have repeatedly ranked RR, HR and systolic BP as the top 3 variables to be monitored. To predict cardiac arrest in ward patients, areas under ROC curves of 0.72, 0.68, 0.55 and 0.48 have been reported for RR, HR, systolic BP, and temperature, respectively (Churpek et al. 2012). In a recent study including >250,000 patients and using machine learning methods for predicting clinical deterioration in ward patients (Churpek et al. 2016), RR had the highest “weight” in the predictive algorithm followed by HR, systolic BP, temperature, and SpO2 (Figure 1). In line with these observations, the National Institute for Health and Care Excellence in the UK stated that “RR is the best marker of a sick patient and is the first observation that will indicate a problem or deterioration in condition” (nice.org.uk/guidance/CG50).
Current options for continuous ward monitoring
Several methods have been proposed for the automatic estimation of RR in ward patients. They are mainly based on capnographic, acoustic, thoracic impedance and piezo-electric techniques (Michard et al. 2017). Capnographic sensors detect expired CO2 and are the reference to measure RR in mechanically ventilated patients. In the context of ward monitoring, they are part of tethered monitoring systems sometimes poorly tolerated by spontaneously breathing patients. Acoustic sensors are better tolerated, but measurements may be influenced by speaking and swallowing (Mimoz et al. 2012). Respiration induces changes in electrical thoracic impedance that can be analysed to measure RR. Chest electrodes are classically used to quantify changes in thoracic impedance and have several advantages including ease of use and patient comfort. However, the reliability of RR measurements depends both on the number and the correct positioning of the electrodes. For patients staying in bed, a contact-free piezo electric sensor, left under the mattress, has been used with success to simultaneously monitor RR and HR (Zimlichman et al. 2012). Brown et al. (2014) monitored medico-surgical inpatients with such a system and reported a significant decrease in the number of calls for cardiac arrest.
Heart rate is classically recorded with chest adhesive electrodes. An alternative is the estimation of pulse rate from a pulse oximetry waveform. Of note, the pulse rate may differ from heart rate in case of cardiac arrhythmia and electromechanical dissociation (heart rate without pulse rate). Taenzer et al. used a tethered pulse oximeter to monitor pulse rate and SpO2 in postoperative orthopaedic patients, many of them receiving opioids (Taenzer et al. 2010). They reported a significant decrease in the number of rescue events and transfers to ICU.
Temperature is classically spot-checked by nurses, and lately adhesive patches have been developed to continuously monitor skin temperature (Michard and Sessler 2018). A recent study showed a good agreement between temperature values from an axillary wearable sensor and reference oesophageal measurements during surgery (Pei et al. 2018). We are not aware of any evaluation conducted on the wards.
Blood pressure remains a variable difficult to measure non-invasively and continuously. Several volume clamp and tonometric methods have been developed to measure finger or radial blood pressure, respectively (Michard et al. 2018a). These systems have been designed for the operating room. Other systems, combining chest electrodes (to detect the ECG R wave) and a finger pulse oximeter (to detect a peripheral pulse), are able to predict BP from the estimation of changes in pulse wave transit time. Weller et al. used such a system to monitor BP, HR, RR and SpO2 in neurological and neurosurgical ward patients (Weller et al. 2018). They reported a significant decrease in the number of rapid response team calls.
Further, when several variables are recorded together, they can be combined to calculate a single Early Warning Score (EWS). Several have been proposed, and they all include RR, HR, and systolic BP. Some (e.g. the NEWS and the ViEWS) integrate SpO2 as well. The calculation of EWSs improves the prediction of adverse events (Churpek et al. 2012; Churpek et al. 2016). The use of monitoring systems that automatically calculate EWSs and alert clinicians on a pager as soon as patients deteriorate has been shown to be associated with clinical outcome benefits (Bellomo et al. 2012, Schmidt et al. 2015, Subbe et al. 2017, Heller et al. 2018). Machine learning algorithms using continuous data as input have the potential to better predict clinical deterioration and adverse events than classical EWSs (Hravnak et al. 2011).
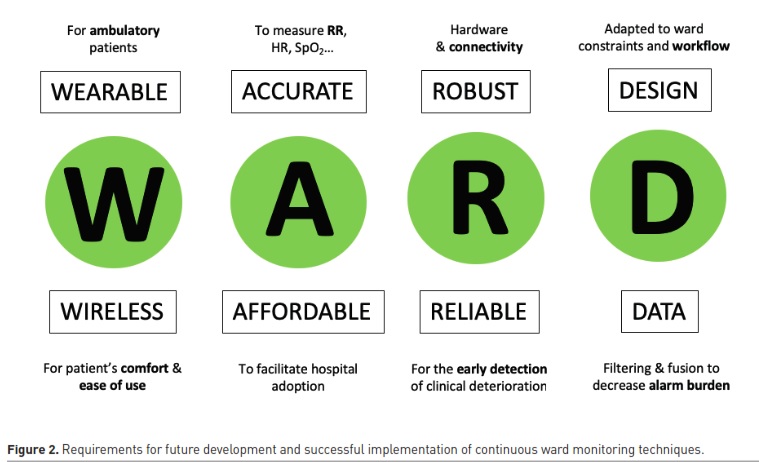
Requirements for future developments and successful implementation
Wireless and wearable sensors
Accuracy and false alarms
Ease of use and impact on workload
Affordability
Conclusion
Finding patients before they rapidly deteriorate and suffer a major adverse event might be the next major opportunity to improve patient safety (Bates and Zimlichman 2015). Thanks to recent hardware (wireless sensors) and software (data filtering and fusion, predictive analytics) innovations, it should become possible to adopt continuous ward monitoring without introducing an unmanageable nurse workload. This, beside the potential clinical benefits (faster recovery, less ICU transfers, and fewer unexpected deaths), could also lead to some logistic (more free beds for new admissions) and economic (shorter hospital stays, fewer complications) benefits. Clinical studies are needed to further investigate the clinical and economic impact of wearable and wireless sensors on medical and surgical wards, and better characterise which patients may benefit the most from these monitoring innovations.
Key points
- Hospital wards are where most unexpected deaths occur within institutions
- Continuous monitoring has the potential to improve quality of care in traditionally unmonitored settings
- Wireless and wearable sensors are highly desirable to make continuous monitoring a reality in ambulatory patients
- Measurement accuracy is mandatory for ensuring that any deteriorating patient is not missed
- Ease of use is key to clinical adoption, and monitoring systems should be made seamless and intuitive for users
- Ward monitoring should not increase workload at the hospital level, but rather redistribute it, with more adverse events managed by ward clinicians and less by critical care specialists
Abbreviations
ASER American Society for Enhanced Recovery
BP Blood pressure
ERAS Enhanced Recovery after Surgery
EWS Early Warning Score
HR Heart rate
POQI Perioperative Quality Initiative
RR Respiratory rate
SpO2 Pulse oxygen saturation
Disclosure
Frederic Michard is the founder and managing director of MiCo, a Swiss consulting firm. Tong J Gan is the founding president of the American Society for Enhanced Recovery (ASER), the president of Perioperative Quality Initiative (POQI) and a consultant for Medtronic. Rinaldo Bellomo has been a consultant for Philips.
References:
Abenstein JP, Narr BJ (2010) An ounce of prevention may equate to a pound of cure: can early detection and intervention prevent adverse events? Anesthesiology, 112:272-3.
Bates DW, Zimlichman E (2015) Finding patients before they crash: the next major opportunity to improve patient safety. BMJ Qual Saf, 24:1-3.
Bellomo R, Ackerman M, Bailey M, et al. (2012) A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med, 40: 2349–61.
Breteler MJM, Huizinga E, van Loon K, et al. (2018) Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: a clinical validation study. BMJ Open, 8:e020162.
Brown H, Terrence J, Vasquez P, et al. (2014) Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med, 127:226-32.
Chen L, Dubrawski A, Wang D, et al. (2016) Using supervised machine learning to classify real alerts and artifact in online multisignal vital sign monitoring data. Crit Care Med, 44:e456-63.
Churpek MM, Yuen TC, Huber MT, et al. (2012) Predicting cardiac arrest on the wards. A nested case-control study. Chest, 141:1170-1176.
Churpek MM, Yuen TC, Winslow C, et al. (2016) Multiem comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med, 44:368-374.
Granholm A, Pedersen NE, Lippert A, et al. (2016) Respiratory rate measured by a standardised clinical approach, ward staff, and a wireless device. Acta Anesth Scand, 60: 1444-52.
Heller AR, Mees ST, Lauterwald B, et al. (2018) Detection of deteriorating patients on surgical wards outside the ICU by an automated MEWS-based early warning system with paging functionality. Ann Surg, doi: 10.1097/SLA.0000000000002830.
Hravnak M, Devita MA, Clontz A, et al. (2011) Cardiorespiratory instability before and after implementing an integrated monitoring system. Crit Care Med, 39 :65-72.
L’Her E, Dias P, Gouillou M, et al. (2017) Automatic versus manual oxygen administration in the emergency department. Eur Respir J, 50:pii 1602552.
Michard F, Mountford WK, Krukas MR, et al. (2015) Potential return on investment for implementation of perioperative goal-directed fluid therapy in major surgery: a nationwide database study. Perioper Med, 4:11.
Michard F, Gan TJ, Kehlet H (2017) Digital innovations and emerging technologies for enhanced recovery programmes. Br J Anaesth, 119:31-39.
Michard F, Sessler DI (2018) Ward monitoring 3.0. Br J Anaesth, 121:999-1001.
Michard F, Sessler DI, Saugel B (2018a) Non-invasive arterial pressure monitoring revisited. Intensive Care Med, 44:2213-2215.
Michard F, Bellomo R, Taenzer A (2018b) The rise of ward monitoring: opportunities and challenges for critical care specialists. Intensive Care Med, doi: 10.1007/s00134-018-5384-5.
Mimoz O, Benard T, Gaucher A, et al. (2012) Accuracy of respiratory rate monitoring using a non-invasive acoustic method after general anaesthesia. Brit J Anaesth, 108:872-5.
Nolan JP, Soar J, Smith GB, et al. (2014) Incidence and outcome of in-hospital cardiac arrest in the United Kingdom national cardiac arrest audit. Resuscitation, 2014; 85:987-92.
Overdyk FJ, Carter R, Maddox RR, et al. (2007) Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg, 105:412-8.
Pearse RM, Moreno RP, Bauer P, et al. (2012) Mortality after surgery in Europe: a 7 day cohort study. Lancet, 380:1059-65.
Pei L, Huang Y, Mao G, Sessler DI (2018) Axillary temperature, as recorded by the iThermonitor WT701, well represents core temperature in adults having noncardiac surgery. Anesth Analg, 126:833-38.
Slight SP, Franz C, Olugbile M, et al. (2014) The return on investment of implementing a continuous monitoring system in general medical-surgical units. Crit Care Med, 42:1862-8.
Schmidt PE, Meredith P, Prytherch D, et al. (2015) Impact of introducing an electronic physiological surveillance system on hospital mortality. BMJ Qual Saf, 24:10-20.
Subbe CP, Duller B, Bellomo R (2017) Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care, 21:52.
Subbe CP, Kinsella S (2018) Continuous monitoring of respiratory rate in emergency admissions: evaluation of the RespiraSense sensor in acute care compared to the industry standard and gold standard. Sensors, 18:2700.
Sun Z, Sessler DI, Dalton JE, et al. (2015) Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg, 2015; 121:709-715.
Taenzer AH, Pyke JB, McGrath SP, et al. (2010) Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: A before-and-after concurrence study. Anesthesiology, 112:282-287.
Taenzer AH, Blike GT (2012) Postoperative monitoring – The Dartmouth experience. Anesthesia Patient Safety Foundation Newsletter: https://www.apsf.org/wp-content/uploads/newsletters/2012/spring/pdf/APSF201206.pdf
Turan A, Chang C, Cohen B, et al. (2019) The incidence, severity and detection of blood pressure perturbations after abdominal surgery. Anesthesiology, in press.
Vincent JL, Einav S, Pearse R, et al. (2018) Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol, 35:325-333.
Weenk M, van Goor H, Frietman B, et al. (2017) Continuous monitoring of vital signs using wearable devices on the general ward: pilot study. JMIR Mhealth Uhealth, 5:e91.
Weller RS, Foard KL, Harwood TN (2018) Evaluation of a wireless, portable, wearable multi-parameter vital signs monitor in hospitalized neurological and neurosurgical patients. J Clin Monit Comput, 32:945-51.
Zimlichman E, Szyper-Kravitz M, Shinar Z, et al. (2012) Early recognition of acutely deteriorating patients in non-intensive care units: assessment of an innovative monitoring technology. J Hosp Med, 7:628-33.