Sepsis is a Medical Emergency Requiring Immediate Diagnosis & Treatment
Pancreatic Stone Protein (PSP) on the abioSCOPE® is the Earliest Marker of Sepsis
Sepsis is a major healthcare burden claiming more than 11 million lives per year, one death every 2.8 seconds1.
It is caused by a dysregulated host response to infection which can progress to multiple organ dysfunction, septic shock and death.
It's a medical emergency that requires immediate diagnosis.
Unfortunately, current standards of care often lead to sepsis being diagnosed too late.
 The clinical signs and symptoms of sepsis are generic and non-specific, making it extremely challenging to timely identify.
The clinical signs and symptoms of sepsis are generic and non-specific, making it extremely challenging to timely identify.
The availability of an early and accurate biomarker at the patient's bedside is key to enabling faster treatment decisions, reduce mortality and lower sepsis-related healthcare costs.
Early Sepsis Detection Up To 72 Hours Before The Standard Of Care In 5 Min*
The PSP Test on the abioSCOPE® Can Save Millions of Lives
The IVD CAPSULE PSP on the abioSCOPE® is the first CE-marked in vitro diagnostic test to enable fast, reliable and early sepsis detection at the point-of-care from a single drop of blood in only 5 minutes*.

A multicentric study published recently in Critical Care, proves that bedside measurement of PSP on the abioSCOPE® clearly correlates with the onset of sepsis, enabling personalized clinical management of patients in the ICU3 .
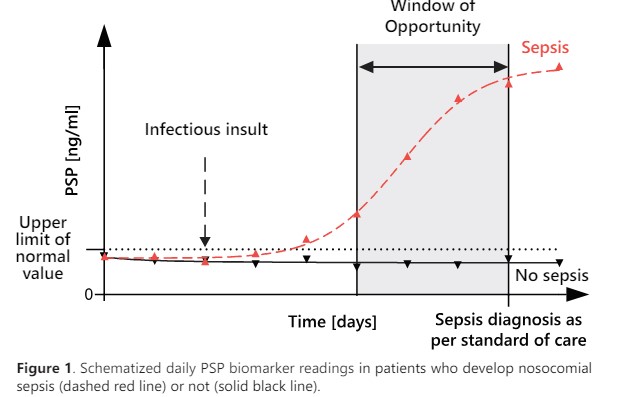 An increasing PSP concentration in the days preceding the clinical diagnosis of sepsis, offers a unique window of opportunity for clinicians to initiate timely the right treatment.
An increasing PSP concentration in the days preceding the clinical diagnosis of sepsis, offers a unique window of opportunity for clinicians to initiate timely the right treatment.
IVD Capsule on the Abioscope Designed for On-Demand Use in the ICU
Scope of Use for the PSP Biomarker in the Diagnosis of Sepsis in Adults
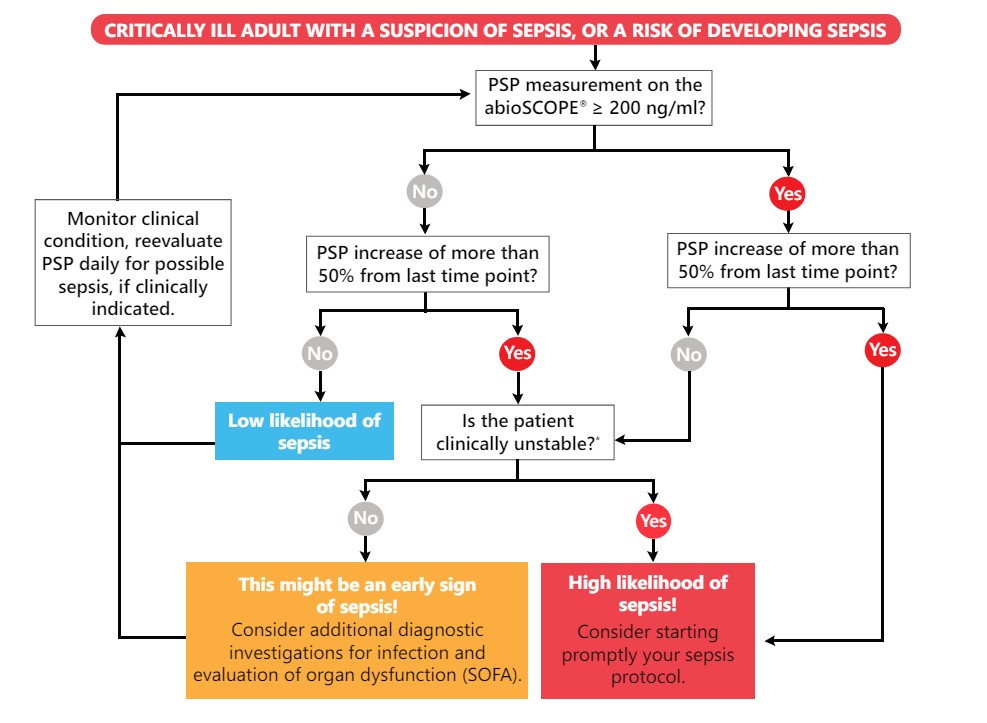
Compact, robust, and intuitive to use, Abionic’s PSP test on the abioSCOPE® is fully compatible with hospital information systems and fits seamlessly into the workflow. When sepsis is suspected, an immediate access to reliable test results is essential. Serial bedside PSP measurements every 24 hours can aid the clinical management and early identification of sepsis in patients at risk. A constantly low PSP value is also a strong indicator of a patient's stability, supporting the decision to not start or withhold unnecessary antibacterial treatment.
Decision Tree for the Interpretation of Serial PSP Measurements
Clinical Evidence
Early Diagnosis of Sepsis in Hospitalized Patients
It is important for intensive care physicians to be able to differentiate between patients suffering from a systemic inflammatory response without infection, compared to those suffering from sepsis. This differential diagnosis is imperative to administer the appropriate treatment.
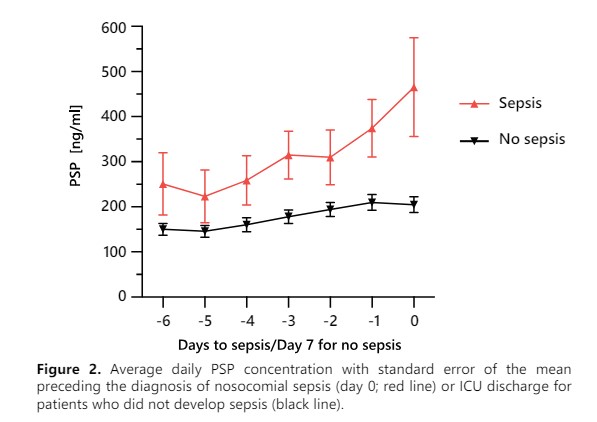 The multicentric study on critically ill patients published in Critical Care, showed that PSP was the only biomarker able to identify sepsis 72 hours before clinical diagnosis according to an external adjudication committee (Fig. 2)3. Providing a large window of opportunity to adapt the patient’s clinical management.
The multicentric study on critically ill patients published in Critical Care, showed that PSP was the only biomarker able to identify sepsis 72 hours before clinical diagnosis according to an external adjudication committee (Fig. 2)3. Providing a large window of opportunity to adapt the patient’s clinical management.
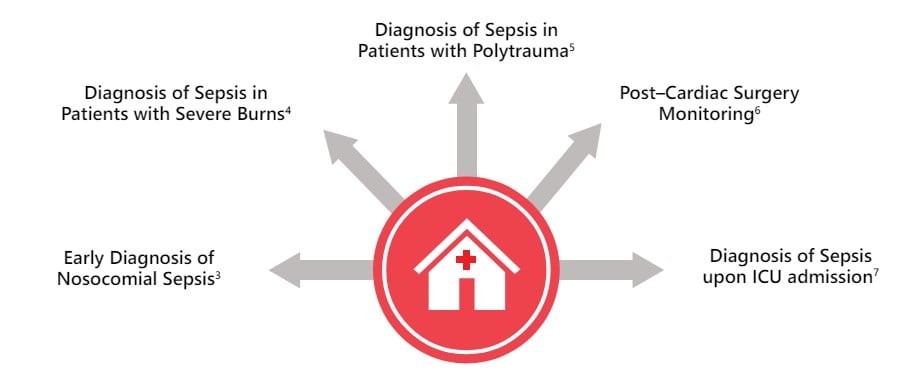
PSP shows similar performances that have been previously reported in studies looking at a variety of critically ill patients, including those with severe burns4 , polytrauma5 , post-cardiac surgery6 and on admission to the intensive care unit (ICU)7.
In addition to individual studies, a large meta-analysis including more than 600 patients also confirms the high diagnostic performance of PSP for diagnosing infection in the ICU and emergency department (ED), with an accuracy of 81%8.
Patient Case Report
Patient history
A 71-year-old male patient was hospitalized for a traumatic brain injury, which required immediate ICU admission with invasive mechanical ventilation.

Biomarkers
The patient’s PSP, CRP, and PCT levels were relatively low on admission. The CRP level was non-specifically elevated from day 2 onward, peaking on day 4. The PCT level was <0.2 ng/ml through day 10, then only increased later on day 11 and on day 12.
PSP remained stable and low until day 7, after which, it began to increase and eventually surpassed the 200 ng/ml cut-off. The continuous increase in the PSP concentration between days 7 and 10 was associated with the development of bacteremia, and anticipated the diagnosis of sepsis by >72h.
Unique Nanotechnology-Based Platform
Abionic’s Patented Nanofluidic Immunoassay Revolutionizes Point-of-Care Diagnostics
 Abionic’s technology enables quantitative results for up to 14 specific parameters in a single capsule.
Abionic’s technology enables quantitative results for up to 14 specific parameters in a single capsule.
Molecules are forced into a nanochannel, limiting their travel distance to a few hundred nanometers and reducing incubation time to 2 minutes9.
PSP level can thus be efficiently quantified within an ultra short assay time, with high precision and accuracy on a closed, small, easy-to-operate platform, providing lab quality results at the point-of-care.
The abioSCOPE®: True Game Changer for the Future of Diagnostics


Source & Image Credit: Abionic
References:
- Global Sepsis Alliance; https://www.global-sepsis-alliance.org/sepsis
- Improving time to antibiotics and implementing the "Sepsis 6". McGregor C. et al., BMJ Open Quality. 2014
- Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: a prospective multicentric study. Pugin et al., Crit Care. 2021 Apr 20
- Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Klein et al., Annals of Surgery. 2020
- Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Keel et al., Crit Care Med. 2009
- Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique. Klein et al., PloS one, 2015
- Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Llewelyn et al., Crit Care. 2013
- Pancreatic Stone Protein for the Diagnostic of Infection. Individual Patient Level Meta-analysis. Prazak et al., Crit Care. 2021
- Nanofluidics Drives Point-of-care Technology for on the Spot Protein Marker Analysis with Rapid Actionable Results. Putallaz, L., Bogaard, P. V. D., Laub, P. & Rebeaud, F. Journal of Nanomedicine & Nanotechnology 2019
* IVD CAPSULE PSP on the abioSCOPE® measurment time: 5 minutes; Total Assay time: 7.5 minutes






