ICU Management & Practice, Volume 17 - Issue 3, 2017
Reviews the latest evidence evaluating physical rehabilitation in the intensive care unit setting and future directions for the field.
Survivors of critical illness frequently experience poor physical outcomes, including persistent impairments in muscle strength, exercise capacity and physical function (Pfoh et al. 2016; Herridge et al. 2011; Dinglas et al. 2017; Fan et al. 2014b). In this article, we review these impairments and recent clinical trials evaluating physical rehabilitation during critical illness as a potential means to improve these outcomes, and conclude with considerations for future studies in the field (Figure 1).
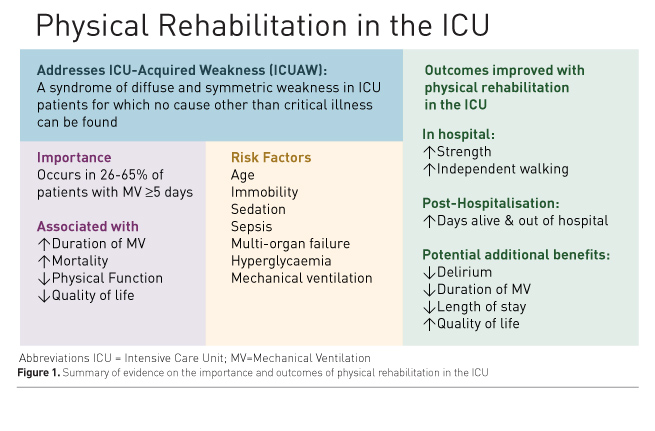
Background: what is intensive care unit (ICU)-acquired weakness?
ICU-acquired weakness (ICUAW) is a syndrome of diffuse and symmetric muscle weakness for which no cause other than critical illness can be found (Stevens et al. 2009). Weakness is defined based on physical examination of muscle strength, in an alert and cooperative patient, using the Medical Research Council (MRC) scale. A MRC sum score of <48 (range: 0-60, maximum = 60) is consistent with ICUAW.
Muscle weakness consistent with ICUAW occurs in 11% of all patients admitted to an ICU for ≥1 day (Nanas et al. 2008), with a higher prevalence of 26-65% in patients mechanically ventilated for ≥5 days (Ali et al. 2008; Sharshar et al. 2009; Dinglas et al. 2017). Loss of muscle mass occurs quickly during critical illness. Compared to the day of ICU admission, cross-sectional area of the rectus femoris muscle decreased by 18% at day 10, with necrosis seen in 54% of muscle biopsies (Puthucheary et al. 2013).
Patients with ICUAW experience worse in-hospital and post-hospitalisation outcomes. Among patients requiring mechanical ventilation for ≥5 days, ICUAW is associated with a 2-fold increase in duration of mechanical ventilation (De Jonghe et al. 2002; Ali et al. 2008), and a 2- to 5-fold increased hospital mortality (Sharshar et al. 2009; Ali et al. 2008). One-year mortality almost doubled in a propensity-matched analysis of patients with vs. without ICUAW (30.6% vs. 17.2%, p = 0.015) (Hermans et al. 2014). Two years after surviving ICU admission for acute respiratory distress syndrome (ARDS), patients with ICUAW achieved only 40% of predicted 6-minute walk distance vs. 60% in those without ICUAW (p <0.01) (Fan et al. 2014b). In the same cohort, patients with vs. without ICUAW demonstrated decreased quality of life (QOL) two years after ARDS (30% vs. 70% of population normative scores on the Short Form 36 Health Survey (SF-36) physical function subscale, p < 0.001) (Fan et al. 2014b). Post-discharge survival over 5 years after ARDS was significantly worse in patients with ICUAW (Dinglas et al. 2017).
Pathology
ICUAW encompasses a variety of muscle and nerve disorders, which may overlap, including critical illness polyneuropathy (CIP), critical illness myopathy (CIM) and disuse atrophy (Stevens et al. 2009). CIP is defined as ICUAW with electrophysiological evidence of a sensorimotor axonal polyneuropathy; CIM is ICUAW with myopathic features on muscle biopsy or electromyography (EMG, recorded during voluntary muscle contraction). CIP and CIM frequently coexist, given common risk factors and potential mediators (Stevens et al. 2009). Pathophysiologically, CIP and CIM are associated with increased inflammatory markers, and microcirculatory and metabolic impairments that are also associated with multi-organ dysfunction syndrome (Batt et al. 2013; Witteveen et al. 2017).
Risk factors
Multiple studies have evaluated patient- and ICU-related risk factors for ICUAW. Older age, immobility, sedation, sepsis, multi-organ failure, hyperglycaemia and mechanical ventilation are consistently reported risk factors for ICUAW (Fan et al. 2014a; Puthucheary et al. 2012; Hermans and Van den Berghe 2015; de Jonghe et al. 2009). The most readily modifiable risk factors are immobility, sedation and hyperglycaemia. Steroids and neuromuscular blocking agents have been reported as risk factors (Hermans and Van den Berghe 2015; Needham et al. 2014), but a causal association is not certain (Puthucheary et al. 2012), given that immobilisation and sedation are confounders in most analyses (deBacker et al. 2017; Fan et al. 2014b). While difficult to evaluate in ICU patients, pre-ICU physical status appears to be an important factor for ICUAW and should be considered when evaluating a patient’s risk for ICUAW (Batt et al. 2013; Latronico et al. 2017; Puthucheary and Denehy, 2015).
You might also like:Early Mobilisation in ICU: From Concept to Reality - Four Steps to Change Patient Outcomes
Evidence: clinical trials evaluating physical rehabilitation
in the ICU
Strength and physical function
The most direct effects of physical rehabilitation in the ICU may be on strength and physical functioning. A recent meta-analysis reported a significant improvement in muscle strength, measured by the MRC sum score, at ICU discharge (pooled mean difference 8.6, 95% CI 1.4-15.9, p = 0.02) and increased probability of walking without assistance at hospital discharge (OR 2.1, 95% CI 1.2-3.8, p=0.01) in rehabilitation intervention vs. control groups (Tipping et al. 2017). Growing evidence suggests that these improvements in strength and physical function may be greater when rehabilitation is initiated earlier. For instance, a randomised controlled trial (RCT) of physical and occupational therapy (PT, OT) interventions, started at a median of 1.5 days after intubation, vs. usual care (with PT and OT started at a median of 7.4 days after intubation) significantly increased return to independent functional status and walking at hospital discharge (59% vs. 35%, p = 0.02) (Schweickert et al. 2009). Similarly, two additional trials of early goal-directed mobilisation vs. usual care reported a doubling of the proportion of patients walking by ICU discharge (Hodgson et al. 2016; Schaller et al. 2016). By contrast, a RCT of more vs. less intensive PT interventions, beginning a median of 8 days after intubation, found no difference in functional status at 28 days (Moss et al. 2015).
Delirium
Several randomised trials have demonstrated an improvement in delirium with rehabilitation in the ICU. Early intervention by PT and OT, delivered during daily sedation interruption, resulted in a 50% decrease in delirium duration compared to very similar sedation (i.e., daily sedation interruption) with usual care rehabilitation therapy (Schweickert et al. 2009). ICU delirium-free days by day 28 increased by 3 days in patients managed with early, goaldirected mobilisation vs. usual care (Schaller et al. 2016). Notably, there was no difference in delirium incidence or duration in a RCT of standardised rehabilitation therapy vs. usual care where there was no sedation protocol and sedation levels that commonly prohibited active physical therapy interventions, which may have contributed to the lack of benefit (Morris et al. 2016). A RCT evaluating interventions led by OT (without additional PT involvement) vs. usual care, reported a dramatic decrease in delirium incidence from 20% to 3% in non-mechanically ventilated patients (Álvarez et al. 2017). Finally, pre-post evaluations of quality improvement bundles, including combined sedation and rehabilitation interventions, have resulted in marked reductions in delirium, although it is impossible to isolate the effect of the rehabilitation component in these studies (Balas et al. 2014; Needham and Korupolu 2010; Smith and Grami 2016).
Duration of mechanical ventilation and length of stay
In a recent systematic review, 3 of 11 RCTs reported significant 1.7-5.8 day decreases in duration of mechanical ventilation (Schweickert et al. 2009; Dong et al. 2016; Dong et al. 2014). Of 13 studies that evaluated ICU length of stay, 10 reported decreases (Tipping et al. 2017), but only 2 reported data that were not potentially confounded by mortality. These studies both found significant reductions of 2.5-5.1 ICU days in intervention vs. control groups (p <0.05) (Yosef-Brauner et al. 2015; Dong et al. 2014).
Mortality and post-discharge status
There is no difference in mortality at ICU discharge, hospital discharge, or 6-month followup in existing RCTs. However, “days alive and out of the hospital at 6 months” were significantly greater with rehabilitation vs. standard care in a recent meta-analysis (mean difference 9.63 days, 95% CI 1.68–17.57, p=0.02) (Tipping et al. 2017).
Quality of life
Physical function and role physical are 2 domains of the SF-36 QOL survey. No differences were seen in these domains at 6 months after ICU admission, although differences were seen in patient subgroups. Early rehabilitation (within 3 days of ICU admission, 1 study) vs. control group increased the SF-36 physical function domain score (mean difference 22 points, p = 0.04) (Kayambu et al. 2013); late rehabilitation (2 studies) vs. control groups was not different (Tipping et al. 2017). The SF-36 role physical domain improved with high-dose (>30 minutes active rehabilitation daily, 2 studies) vs. control groups (mean difference 31 points, p = 0.001); low-dose rehabilitation (1 study) vs. control group was not different (Tipping et al. 2017).
Safety
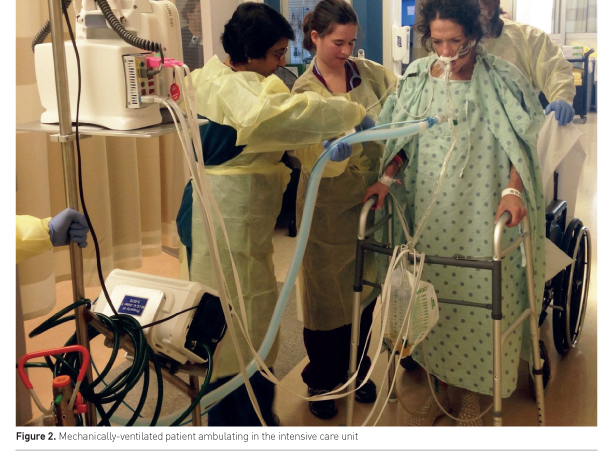
Physical rehabilitation of critically ill patients (Figure 2) is safe (Tipping et al. 2017; Nydahl et al. 2017). A large systematic review of 22,351 mobilisation sessions delivered in 7,546 ICU patients, from a combination of observational and clinical trials, demonstrated a rare frequency of events. Potential safety events, defined as a clinical deterioration or event exceeding a study’s safety limit, occurred in only 2.6% of sessions. Events of consequence, defined as being events associated with cessation of a mobility session, adverse health consequence or requirement of additional therapy, occurred in only 0.6% of sessions. Most potential safety events involved a haemodynamic change or oxygen desaturation that resolved with pause or cessation of mobility. Notably, removal of medical devices and falls were rare, including dysfunction or removal of an intravascular catheter (0.2% of sessions), endotracheal tube removal (0.01%) and falls (0.07%) (Nydahl et al. 2017).
Future directions
The most pressing questions for consideration in future studies in the field include the optimal type and dose of rehabilitation interventions, timing of initiation of intervention and ICU patient sub-populations (Denehy et al. 2017). While PTs may mobilise patients to a higher level than nurses (Garzon-Serrano et al. 2011), nurses can provide clinically beneficial mobility interventions, as clearly demonstrated in a recent RCT (Schaller et al. 2016). There are many types of interventions to be considered in future studies, including functional mobility, strengthening and use of relevant technology and equipment (e.g., in-bed cycle ergometry, neuromuscular electrical stimulation, tilt tables, interactive video games, hydrotherapy), along with consideration of potentially synergistic interventions with rehabilitation, such as nutritional supplementation (Needham et al. 2009; Sommers et al. 2017; Arabi et al. 2017; Heyland et al. 2015).
There is emerging evidence demonstrating the benefit of earlier vs. later initiation of rehabilitation in the ICU, which should be considered when designing future trials and implementing rehabilitation as part of clinical practice in the ICU. Most studies have had broad eligibility criteria, but it is possible that patients’ pre-ICU baseline status (e.g., frailty and comorbidity) or ICU-based diagnosis (e.g., sepsis) may better identify patients who are most likely to benefit from rehabilitation (Puthucheary and Denehy 2015).
Finally, more fully understanding the effects of ICU-based rehabilitation is limited by heterogeneity in reporting among the published reports. Future studies should adopt standardised reporting methods of intervention, potential safety events and outcome measures, including separately reporting outcomes for survivors and non-survivors where appropriate (Hoffmann et al. 2014; Slade et al. 2016; Connolly et al. 2017; Needham et al. 2017).
Conclusions
Muscle wasting and weakness commonly develops within days of ICU admission, with effects on survival and physical functioning lasting for years post-discharge. Early-onset physical rehabilitation is a safe intervention in ICU patients that, based on existing RCTs, improves strength and physical functioning, and may improve delirium in the ICU as well as in-hospital and post-hospitalisation healthcare resource utilisation.
Conflict of interest
Dale M. Needham is a principal investigator on a NIH-funded multi-site randomised trial evaluating nutrition and exercise in acute respiratory failure and, related to this trial, is currently in receipt of an unrestricted research grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Device. The other authors declare that they have no conflict of interest.
Abbreviations
ARDS acute respiratory distress syndrome
CIM critical illness myopathy
CIP critical illness polyneuropathy
EMG electromyography
ICU intensive care unit
ICUAW intensive care unit-acquired weakness
MRC Medical Research Council
OT occupational therapy
PT physical therapy
QOL quality of life
RCT randomised controlled trial
SF-36 Short Form 36 Health Survey
References:
Álvarez EA, Garrido MA, Tobar EA et al. (2017) Occupational therapy for delirium management in elderly patients without mechanical ventilation in an intensive care unit: A pilot randomized clinical trial. J Crit Care, 37: 85-90.
Arabi YM, Casaer MP, Chapman, M et al. (2017) The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med, 3 Apr. doi: 10.1007/s00134-017-4711-6. [Epub ahead of print]
Balas MC, Vasilevskis EE, Olsen KM et al. (2014) Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit. Care Med, 42: 1024-36.
Batt J, Dos Santos CC, Cameron JI et al. (2013) Intensive care unit–acquired weakness. Am. J. Respir. Crit. Care Med, 187: 238-246.
Connolly B, Blackwood B, Hart N et al. (2017) Development of a core outcome set for trials of physical rehabilitation after critical illness [Online]. COMET Initiative. [Accessed: 27 May 2017]
Available from http://www.comet-initiative.org/studies/details/288
De Jonghe B, Lacherade JC, Sharshar T et al. (2009) Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med, 37: S309-15.
De Jonghe B, Sharshar T, Lefaucheur JP et al. (2002) Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA, 288: 2859-67.
Debacker J, Hart N, Fan E (2017) Neuromuscular blockade in the 21st century management of the critically ill patient. Chest, 151: 697-706.
Denehy L, Lanphere J, Needham DM (2017) Ten reasons why ICU patients should be mobilized early. Intensive Care Med, 43: 86-90.
Dinglas VD, Friedman LA, Colantuoni E et al. (2017) Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med, 45: 446-53.
Dong Z, Yu B, Zhang Q et al. (2016) Early rehabilitation therapy is beneficial for patients with prolonged mechanical ventilation after coronary artery bypass surgery. Int Heart J, 57: 241-6.
Dong ZH, Yu BX, Sun YB et al. (2014) Effects of early rehabilitation therapy on patients with mechanical ventilation. World J Emerg Med, 5: 48-52.
Fan E, Cheek F, Chlan L et al. (2014a) An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med, 190: 1437-46.
Fan E, Dowdy DW, Colantuoni E et al. (2014b) Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med, 42: 849-59.
Garzon-Serrano J, Ryan C, Waak K et al. (2011) Early mobilization in critically ill patients: patients' mobilization level depends on health care provider's profession. PM R, 3: 307-13.
Hermans G, Van den Berghe G. (2015) Clinical review: intensive care unit acquired weakness. Crit Care, 19: 274.
Hermans G, Van Mechelen H, Clerckx B et al. (2014) Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med, 190: 410-20.
Herridge MS, Tansey CM, Matté A et al. (2011) Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med, 364: 1293-304.
Heyland DK, Stapleton RD, Mourtzakis M. et al. (2015) Combining nutrition and exercise to optimize survival and recovery from critical illness: conceptual and methodological issues. Clin. Nutr, 35: 1196-206.
Hodgson, C. L, Bailey, M, Bellomo, R et al. (2016. A Binational Multicenter Pilot Feasibility Randomized Controlled Trial of Early Goal-Directed Mobilization in the ICU. Crit Care Med, 44, 1145-52.
Hoffmann TC, Glasziou PP, Boutron I et al. (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348: g1687.
Kayambu G, Boots R, Paratz J (2013) Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit. Care Med, 41: 1543-54.
Latronico N, Herridge M, Hopkins RO et al. (2017) The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med, 13 Mar. doi: 10.1007/s00134-017-4757-5. [Epub ahead of print]
Morris PE, Berry MJ, Files DC et al. (2016) Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA, 315: 2694-702.
Moss M, Nordon-Craft A, Malone D et al. (2015) A randomized trial of an intensive physical therapy program for acute respiratory failure patients. Am. J. Respir. Crit. Care Med, 193: 1101-10.
Nanas S, Kritikos K, Angelopoulos E.et al. (2008) Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand, 118: 175-81.
Needham DM, Korupolu R (2010) Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil, 17: 271-81.
Needham DM, Sepulveda KA, Dinglas VD et al. (2017) Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med, 24 May. doi: 10.1164/rccm.201702-0372OC [Epub ahead of print]
Needham DM, Truong AD, Fan E (2009) Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med, 37: S436-41.
Needham DM, Wozniak AW, Hough CL et al. (2014) Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med, 189: 1214-24.
Nydahl P, Sricharoenchai T, Chandra S et al. (2017) Safety of patient mobilization and rehabilitation in the intensive care unit. systematic review with meta-analysis. Ann Am Thorac Soc, 14: 766-77.
Pfoh ER, Wozniak AW, Colantuoni E et al. (2016) Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med, 42: 1557-66.
Puthucheary Z, Rawal J, Ratnayake G et al. ( 2012) Neuromuscular blockade and skeletal muscle weakness in critically ill patients: time to rethink the evidence? Am J Respir Crit Care Med, 185: 911-7.
Puthucheary ZA, Denehy L (2015) Exercise interventions in critical illness survivors: understanding inclusion and stratification criteria. Am J Respir Crit Care Med, 191: 1464-7.
Puthucheary ZA, Rawal J, McPhail M et al. 2013. Acute skeletal muscle wasting in critical illness. JAMA, 310: 1591-600.
Schaller SJ, Anstey M, Blobner M et al. (2016) Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet, 388: 1377-88.
Schweickert WD, Pohlman MC, Pohlman AS et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet, 373: 1874-82.
Sharshar T, Bastuji-Garin S, Stevens RD et al. (2009) Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med, 37: 3047-53.
Slade SC, Dionne CE, Underwood M et al. (2016) Consensus on Exercise Reporting Template (CERT): modified Delphi study. Phys Ther, 96: 1514-24.
Smith CD, Grami P (2016) Feasibility and effectiveness of a delirium prevention bundle in critically ill patients. Am J Crit Care, 26: 19-27.
Sommers J, Wieferink DC, Dongelmans DA et al. (2017) Body weight-supported bedside treadmill training facilitates ambulation in ICU patients: An interventional proof of concept study. J Crit Care, 41: 150-5.
Stevens RD, Marshall SA, Cornblath DR et al. (2009) A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med, 37: S299-308.
Tipping CJ, Harrold M, Holland A et al. (2017) The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med, 43: 171-83.
Witteveen E, Wieske L, van der Poll, T, et al. (2017) Increased early systemic inflammation in ICU-acquired weakness; a prospective observational cohort study. Crit Care Med, 45: 972-9.
Yosef-Brauner O, Adi N, Ben Shahar T et al. (2015) Effect of physical therapy on muscle strength, respiratory muscles and functional parameters in patients with intensive care unit-acquired weakness. Clin Respir J, 9: 1-6.














