ICU Management & Practice, Volume 25 - Issue 3, 2025
Implementing an AI screening tool in our ICU for palliative care consults streamlined early alignment of treatment with patient goals, decreased futile interventions, and demonstrated scalable, value-based, patient-centric critical care delivery.
Introduction
This special edition of Value-Based Critical Care highlights innovative approaches that improve clinical outcomes while ensuring care reflects what matters most to patients in high-acuity environments. Care in the Intensive Care Unit (ICU) inherently exposes patients to invasive procedures, prolonged hospital stays, and outcomes that may not reflect their preferences. Palliative care (PC) interventions can help avoid some of these non-beneficial procedures while providing essential emotional and decision-making support to caregivers and family members during some of their most challenging moments. By incorporating PC into standardised ICU workflows, systems can ensure consistent, patient-centred care and allow care teams to address patient preferences earlier and during treatment, aligning care more closely with patients’ treatment goals (Cox et al. 2018; Courtright et al. 2019; Phillips et al. 2024).
PC in the ICU plays a vital role in addressing the complex needs of critically ill patients and their families by focusing on symptom relief in addition to the emotional, psychological, and spiritual needs and alignment of medical care with individual goals. This is accomplished by providing interdisciplinary care with ICU physicians to ensure patient care is congruent with the patient's values, particularly in complex and critical situations. PC emphasises clear communication and individualised treatment plans to ensure shared decision-making. Advance care planning is essential to this process, specifically when the prognosis is uncertain or interventions may be burdensome and unwanted. Studies have consistently shown that when care is aligned with patient values, both satisfaction and outcomes improve while avoiding unnecessary treatments and without increasing mortality (Bajwah et al. 2020; Aslakson et al. 2014).
Despite growing recognition of the value of PC, the service remains inconsistently applied in the ICU, with referrals often delayed until late in the illness trajectory, limiting meaningful goals-of-care discussions or symptom management options. This underutilisation may be driven by several barriers, including provider variability, time constraints, and a lack of standardised referral criteria (Wysham et al. 2017). A significant barrier is the misconception that initiating PC early signals the end of care or the cessation of therapeutic treatments (Batzler et al. 2024). This misunderstanding contributes to hesitation among clinicians and families despite evidence increasingly supporting that timely PC, particularly when delivered within the first 24 hours of ICU admission, is associated with improved outcomes, reduced hospital mortality, and increased hospice discharges (Romano et al. 2017; Flieger et al. 2020).
Unfortunately, PC consultations are still frequently made late (i.e., 48-72 hours after ICU admission or near the end of life), which may alter the patient’s clinical course (Cox et al. 2018). Aggressive interventions may have already been initiated within this initial time period. Such a delay can limit the opportunity to establish goals of care early on, potentially leading to unnecessary or unwanted treatment. Recognising this gap between evidence and practice, Mayo Clinic (MC) developed and implemented a value-based algorithm to standardise criteria for identifying palliative care needs. Based on clinical and functional variables, this PAL (Palliative) Score represents the percentile of risk (1-100) and identifies patients at high risk of poor outcomes who may benefit from early palliative consultation. By automating the consultation trigger through integration with the electronic health record (EHR), this model minimises reliance on clinician discretion alone and operationalises PC as a proactive - not reactive - component of ICU care. This paper describes our institution’s experience designing, piloting, and embedding this algorithmic approach within ICU workflows. This initiative demonstrates how value-based frameworks can be meaningfully applied to PC, fostering patient-centred care even in the highest acuity ICU settings.
Background
Integrating artificial intelligence (AI) into ICUs holds promise for improving the timely delivery of PC to critically ill patients. Recent work on the development, implementation, and evaluation of an AI-based clinical decision support tool for predicting PC consultations demonstrated significant increases in consultation rates and reductions in readmissions across inpatient units, including cardiology, critical care, and oncology services (Murphree et al. 2021; Wilson et al. 2023). The evaluation demonstrated a 44% increase in consultations and reductions in 60- and 90-day readmissions among patients identified by the AI model.
However, adapting AI-supported trigger tools to the ICU requires addressing unique clinical and sociotechnical challenges. ICU environments are characterised by high acuity, rapid decision-making, and limited access to specialty PC services, making screening for unmet PC needs difficult. Kistler et al.’s (2020) review of PC trigger tools revealed considerable heterogeneity in design and moderate to high risk of bias, with 33% of positively triggered patients receiving PC consultations. This finding highlights that identification alone is insufficient; screening efforts occur within a complex backdrop of system-level barriers constraining action even when the need is recognised. Recent work documents multiple barriers: limited resources, relentless pace of high-acuity care, strained communication channels, absence of local champions, perceived incompatibility with ICU workflows, variability in clinicians’ knowledge and beliefs about PC, as well as structural and cultural challenges, such as negative perceptions of PC (Effendy et al. 2022; Meddick‐Dyson et al. 2024). Together, these factors suggest that using AI to improve PC screening in the ICU must be paired with implementation strategies that address downstream delivery processes to ensure successful implementation.
Several facilitators can counterbalance these obstacles and streamline PC integration. Implementation resistance can be reduced through effective collaboration between ICU and PC teams, targeted education, support by engaged nursing staff that facilitates timely and coordinated delivery, and having clinicians with prior PC exposure, who tend to be more receptive. Strategies include PC clinicians joining daily ICU rounds, local champion advocacy, and visible leadership support to help normalise consultations and foster cultural change. Cultivating trust and a supportive institutional culture ensures that AI-generated prompts are recognised as actionable, clinically valuable information rather than additional noise. These facilitators are prerequisites for successfully implementing AI-driven interventions in critical settings; without them, even the most accurate model is unlikely to translate into timely, patient-centred PC action (Courtright et al. 2020; Effendy et al. 2022; McDarby and Carpenter 2019; Meddick‐Dyson et al. 2024).
Study Aims
Algorithm Development and Adaptation: To describe the development of the PC algorithm in MC Rochester and its adaptation for use in MC Florida, including adjustments for local conditions.
Pilot Testing and Implementation: To outline the pilot testing process and the steps taken to transition the algorithm from pilot to practice change in the ICU.
Evaluating Impact: To assess the impact of the algorithm on patient care, including metrics such as ICU days, alignment with patient wishes, and patient and family experience.
Methods
Overview of the Original Model
We previously developed and deployed a machine learning (ML) model to identify hospitalised patients who may benefit from PC consultation based on structured EHR data. Full details of the model architecture, feature engineering, and deployment strategy can be found in Murphree et al. (2021). In the original deployment, the algorithm was trained on a retrospective cohort of 68,349 inpatient encounters at the MC Rochester campus.
Model Updating
The study population consisted of all Rochester, Florida, Arizona, and MC Health System (MCHS) patients admitted into the hospital between January 1, 2020, and September 1, 2023. Feature selection and model architecture were kept consistent with the original approach described in Murphree et al. (2021), with updates to feature classes based on recommendations from the PC team and insights from deployment and evaluation.
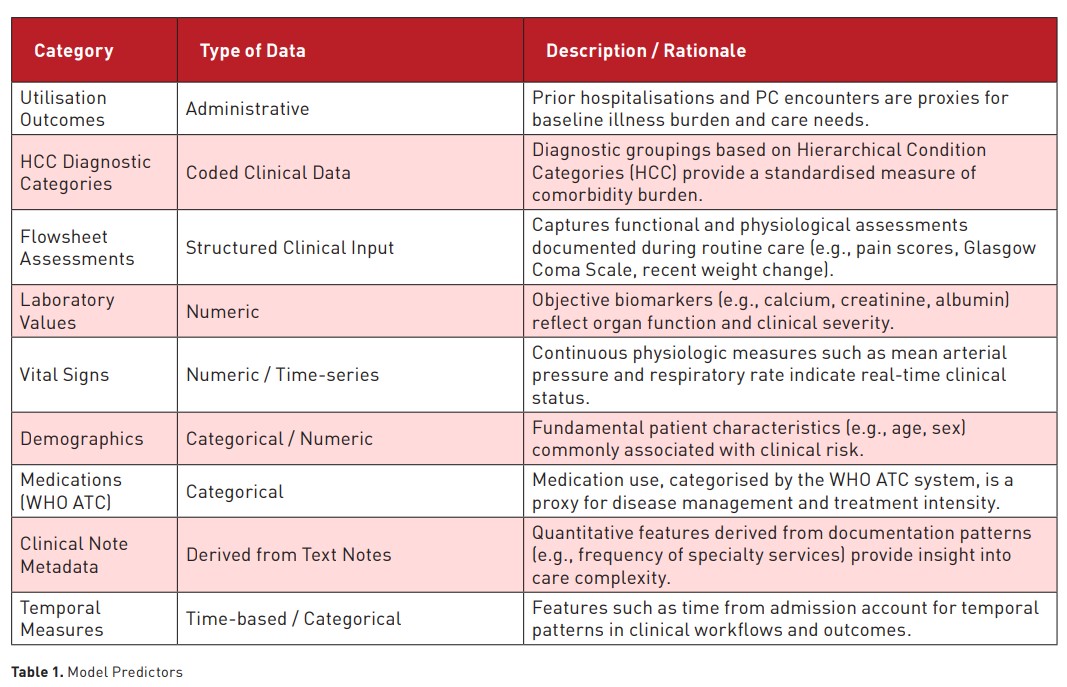
The final predictive model incorporated 269 variables drawn from clinical, demographic, and administrative data sources. These predictors reflected various domains relevant to patient status and care complexity, including prior healthcare utilisation, comorbidities, clinical assessments, laboratory values, and temporal features. Table 1 summarises the major categories of predictors used in the model, along with representative examples and a brief rationale for their inclusion.
Performance of New Version Across ICU Sites
The second phase involved applying the updated model to a subpopulation at a different hospital site within the same health system. Specifically, we evaluated model performance at each new site, particularly for patients who spent any portion of their hospitalisation in the ICU. For the analyses, patients were classified as ‘ICU’ if they were admitted to the ICU at any point during their hospital stay, and model performance was assessed only during the ICU period, with follow-up censored at ICU transfer or discharge.
We evaluated model performance using ROC curves and AUC statistics. Since risk scores varied over time during a patient’s encounter, the maximum score a patient received before an event or discharge was used as their representative risk score. This approach reflected how the model would be used in practice, where an alert is triggered once a patient’s score crossed a threshold at any point.
Prospective Pilot Implementation
In the final phase, we prospectively tested the updated model as part of a pilot implementation within a clinical delivery model. Model predictions were surfaced to the PC team using an adapted display interface, and recommendations were communicated to inpatient teams. We recorded uptake metrics, timeliness of PC consults, and team feedback to assess feasibility, acceptance, and workflow integration. Process outcomes such as consult initiation rate were recorded.
AI Tool Implementation
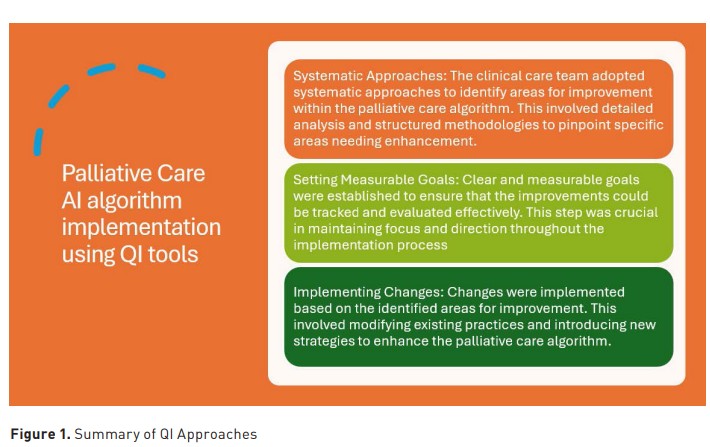
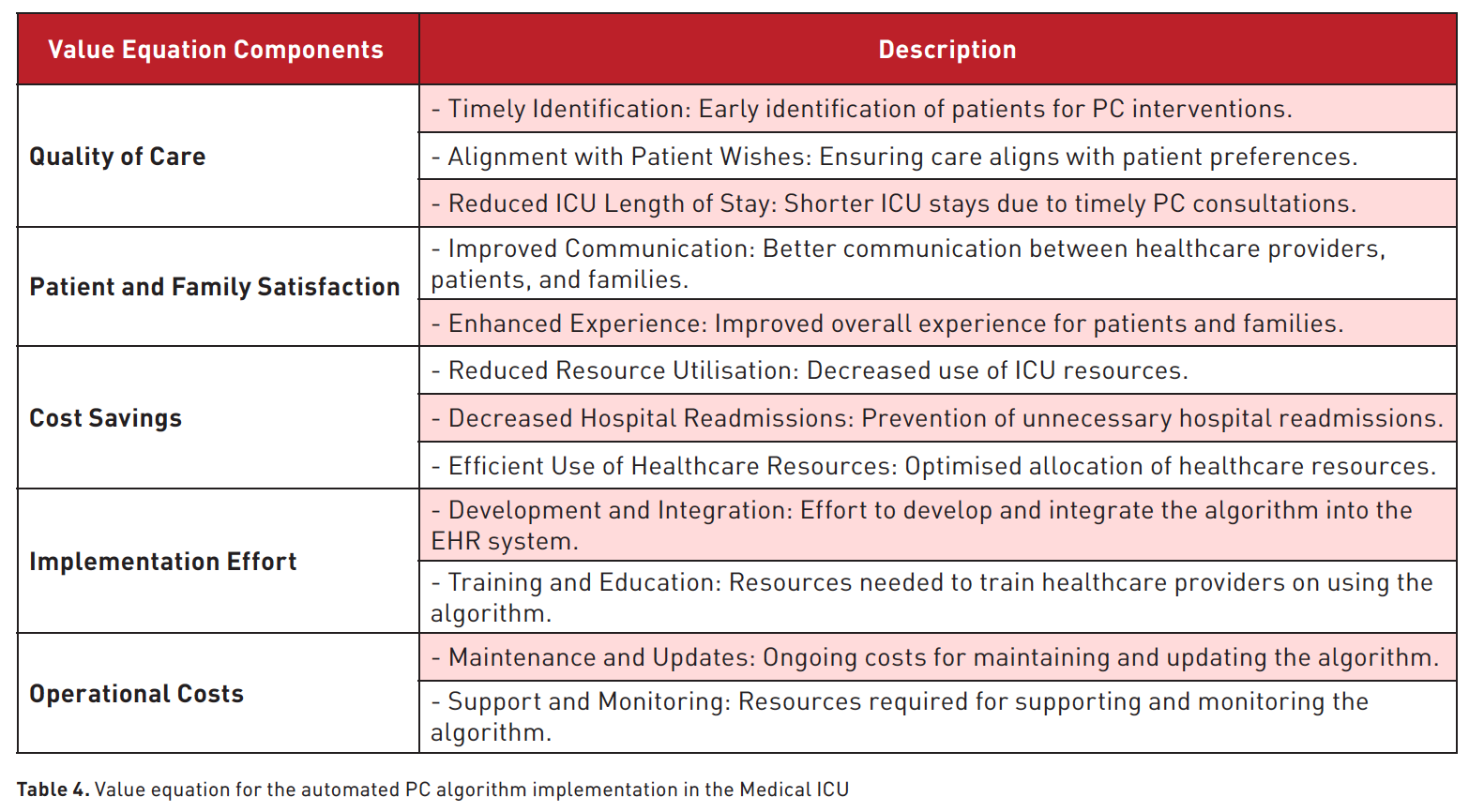
Implementing the AI-driven ICU PC screening process followed a structured daily workflow. The clinical care team utilised various quality improvement (QI) tools and methodologies to streamline processes, improve efficiency, and achieve better outcomes. These QI methodologies, summarised in Figure 1 and Table 4, were crucial in ensuring that the PC algorithm was effectively implemented, ultimately enhancing patient outcomes and aligning care with patient preferences.
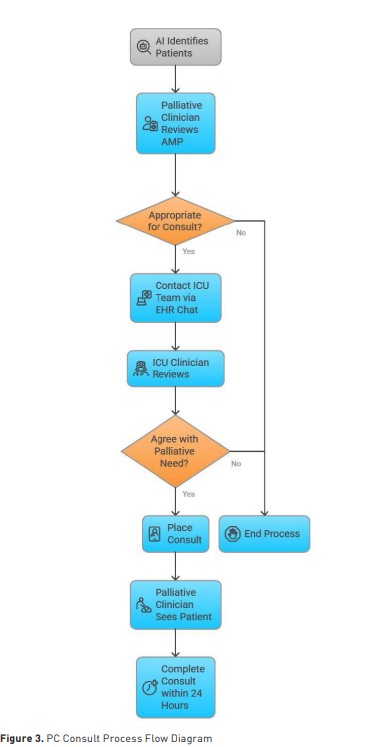
Each morning, the PC provider accessed the AI platform to review ICU patients and identify those with a high palliative score (greater than 70), indicating potentially unmet PC needs. For patients exceeding this threshold, the provider conducted a brief chart review to confirm the appropriateness of palliative involvement. If the provider agreed with the AI-generated recommendation, they notified the ICU clinician of the patient’s high score and potential need for consultation. The ICU clinician subsequently reviewed the patient’s chart and, if in agreement, placed an order for formal palliative consultation. If the ICU clinician disagreed with the recommendation, the process concluded without further action. For patients with an approved consultation, the PC provider conducted a bedside evaluation within 24 hours and communicated recommendations directly to the ICU team to support goal-concordant care planning.
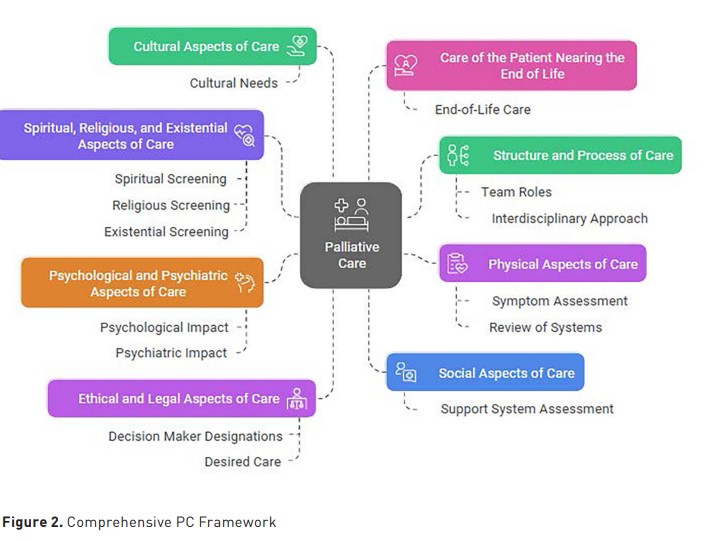
The PC consultations were conducted by clinicians board-certified in Hospice and Palliative Medicine or an Advanced Practice Provider with experience in PC. The consultations included an assessment of symptoms, a review of the goals of care, advance care planning, and caregiver support. The consultation structure aligned with the eight PC domains described in Figure 2 (National Coalition for Hospice and Palliative Care 2018).
Additional members of the PC interdisciplinary team, such as the Chaplain, Social Worker, and Nurse, assessed the patient or provided support as deemed appropriate by the PC clinician who completed the consultation. The consultation documentation was available in the EHR on the same day the patient was seen.
Results
Table 2 presents descriptive statistics for the cohort, stratified by site and ICU status. Table 3 summarises model performance (AUC) across these strata. Notably, the Florida site subgroup, although one of the smaller sites, still had acceptable model performance for moving forward with a pilot (AUC = 0.807, 95% CI: 0.757–0.855).
Between August 2024 and January 2025, the AI tool identified 52 patients in the Medical ICU at MC Florida with a palliative score greater than 70. After a review of the EHR by a PC specialist, 46 (85%) of those patients were deemed to be appropriate for PC based on their critically ill status, medical comorbidities, laboratory values, and need for a PC intervention to discuss goals of care, advance care planning, or life-prolonging measures. The Medical ICU attending physicians agreed to place a consultation for 17 (37%) patients due to a variety of factors, including patient/family preference.
Of the patients seen by the PC team, goals of care were defined, changing the course of their hospitalisation, eight patients changed their code status to DNR/DNI, six patients were discharged to inpatient hospice care, and five patients underwent compassionate withdrawal of life-prolonging measures. Of the two patients discharged home, one was under hospice care.

Among the patients not seen by the PC team, reasons for declining consultation by the ICU team included downgrading from the ICU 5 (17%), ICU team already guiding palliative conversations 3 (10%), no reason given 16 (55%) and other reasons 5 (17%) such as the need for consultation/second opinion from different services, decisions already made to pursue hospice care and family preference. Of note, patients already followed by the palliative medicine team were excluded from the AI scoring system.
Discussion
Healthcare systems increasingly turn to models emphasising value-based care to address challenges and barriers to PC initiation. These models prioritise individualised high-quality treatment that improves outcomes while respecting patient autonomy and ensuring resource stewardship (Rao et al. 2024; Buang et al. 2022). In the ICU, this approach often involves developing and utilising algorithms to identify patients who would benefit from PC consultations early in their stay (Kavalieratos et al. 2016; May et al. 2015). By focusing on providing this patient-centred care, the model reframes care delivery from a volume-based to a value-centric paradigm – to emphasise understanding patient desired outcomes and cost-efficiency (Hall 2014). However, as highlighted by the Institute of Medicine and ethicists alike, achieving this transformation requires a system-wide culture shift, embracing the inherent value of PC as a standard component of serious illness management (Institute of Medicine 2014; Carmichael et al. 2021).
This initiative illustrates how interdisciplinary collaboration and iterative piloting can support the successful deployment of ML tools in critical clinical environments. Building on a previously validated algorithm to identify hospitalised patients who may benefit from PC consultation, we extended the tool to a new hospital site within the same health system, focusing on ICU populations. From our experience in the ICU, this meant weaving structured approaches and integrating the predictive algorithm into both our internal graphic user interface that displays the PAL score and defined workflows to offer a scalable solution. A key initial step was automating EHR consultation triggers, which helped reduce workload and reliance on subjective decision-making to ensure more equitable access to supportive care. Standardised criteria for PC referrals also promote consistency in care delivery, aligning with the principles of value-based care (Adelson et al. 2017).
For the pilot study, we utilised one main provider to be the gatekeeper, reviewing the tool, and the broader PC team were available to hold PC consultations. Training multiple PC providers to review the PAL Score and potentially cross-training the ICU providers so they can assist with the early identification of patients that would benefit from PC are avenues planned for the next steps of the broader implementation. It is important to note that while we don't have dedicated FTE for this role, we do have appropriate staffing to increase the penetration rate in the ICU (i.e., patients seen by PC/ patients admitted to the ICU). The ability to identify and tailor the resources offered toward end-of-life care creates significant value for patients, their families, and the institution by reducing unnecessary interventions and providing essential emotional and decision-making support during critical moments.
While the study focused on early PC consultations with patients seen on the day they were identified as fitting criteria for a PC consult, there are lessons learned that will aid in future implementations. The PAL score did not screen out patients who already had primary PC performed by the Medical ICU team, instances where decisions about patient preferences were outlined, patients who were already receiving goal-concordant care, or patients who were already planned for a withdrawal or de-escalation of life-prolonging measures. Thus, not every patient identified by the AI tool may have needed a consultation performed by a specialist, which required additional resources. Our workflow was designed intentionally for PC providers to act as the gatekeeper, screening scores and subsequently contacting the critical care team, utilising the expertise of the PC team as the initial reviewers of the automated score. As we transition from the pilot phase into wider utilisation, our goal is to encourage early utilisation of PC services as the standard of care based on the PAL Score. Since PC is often misconstrued by patients, families, and even staff, this integrative approach fosters collaboration between PC and ICU providers, positioning them as a unified member of the primary care team.
The updated model incorporated enterprise-level data sources and refinements based on frontline feedback, allowing for improved compliance with clinical priorities. Importantly, although predictive performance was acceptable, prospective pilot results suggested meaningful room for improvement in actual consult uptake and workflow integration. These findings reinforce a central tenet of clinical AI deployment: generalisability is not achieved solely through technical model robustness but through continuous, site-specific iteration. Differences in patient demographics, documentation patterns, and care culture between institutions necessitate recalibration and thoughtful adaptation, even within a shared health system.
Our work showcased that algorithm performance in isolation is insufficient. Effective ML tools must be delivered through mechanisms that integrate with existing workflows and meet the needs of local care teams as well as the patients. These implementation lessons parallel prior findings that clinician trust, alert timing, and workflow fit are critical facilitators of ML impact (Effendy et al. 2022; Meddick‐Dyson et al. 2024). An important point to remember is that even the most accurate models can falter if not embedded within a responsive and well-supported clinical pathway. Identifying patients is only the first step; ensuring timely, effective response requires alignment across teams, clear communication protocols, and cultural readiness.
Effective implementation and continuous improvement of the PC algorithm in the ICU required a structured evaluation and feedback process. These processes should include tracking key metrics like ICU length of stay and timeliness of PC consultations, gathering stakeholder feedback through surveys and interviews, and conducting regular reviews to assess performance and address challenges. Ongoing staff training sessions will ensure familiarity with the algorithm and identify areas for updates, while precise reporting mechanisms will help highlight successes and suggest enhancements. Collaboration between ICU staff, PC specialists, and other departments is crucial to adapting the algorithm to evolving patient care needs and fostering a culture of continuous improvement within the ICU.
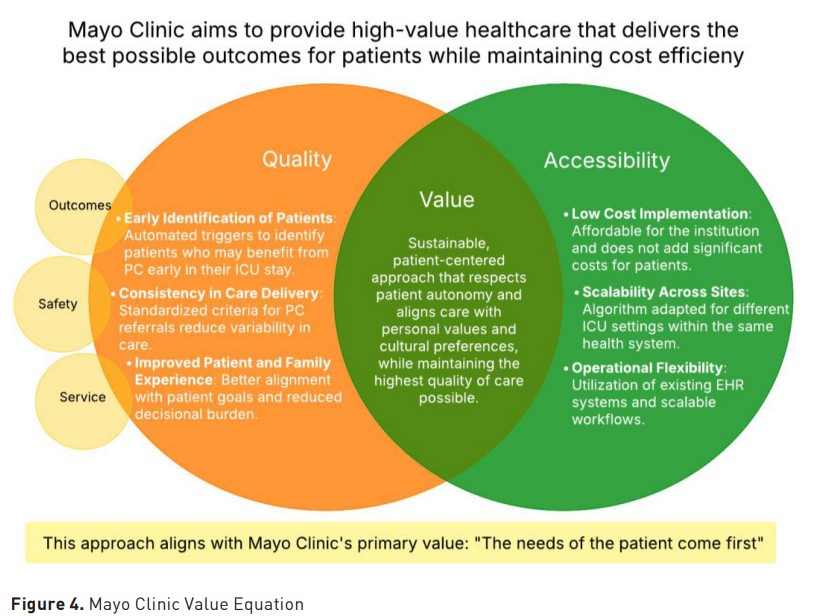
Mayo Clinic's value equation Value = Quality (Outcomes, Safety, Service / Cost (Figure 4) is a fundamental concept that guides our approach to delivering high-quality healthcare. This equation aligns with Mayo Clinic’s primary value, which emphasises that value increases when the quality of care is improved and when the cost of delivering that care decreases.
The timing of these consultations may vary depending on the availability of PC teams and the specific needs of patients. Future research should explore the impact of varying consultation timings and address potential barriers to timely PC interventions. While the study demonstrated positive outcomes regarding hospital mortality and discharges to hospice, it did not extensively explore the perspectives of patients and their families regarding PC interventions. Understanding their experiences and satisfaction with the care provided is essential for improving the overall quality of PC in the ICU. Qualitative assessment could capture patient and family perspectives. Another opportunity to conduct additional research would be to conduct a detailed economic evaluation of the cost savings associated with early PC consultations, which may help encourage wider adoption of standardising criteria around PC offers in the ICU. Future research might also explore the feasibility and impact of making PC a standard of care in the medical ICU, with the team integrated into the clinical care setting.
Normalising PC involvement may increase acceptance by ICU providers, patients, and families. This approach can ensure that all patients receive timely PC interventions, improving overall patient outcomes and satisfaction. This project represents progress toward scalable, ML-driven PC identification while raising critical questions about long-term sustainability. The technical and social infrastructure required to maintain and evolve these tools is considerable. Current funding streams often emphasise innovation over maintenance, leaving institutions with few mechanisms to support ongoing monitoring, recalibration, and clinician engagement. Sustained impact will depend on shifting funding and institutional mindsets to treat AI tools not as static products but as evolving components of clinical quality improvement. Supporting iterative add-on initiatives and embedding AI model monitoring into routine operations will ensure these systems continue delivering value over time.
Conclusion
Delivering high-quality care in the ICU increasingly demands alignment with the principles of value-based medicine, prioritising interventions that not only prolong life but enhance the quality of life, support patient autonomy, and reduce non-beneficial care. Critically ill patients benefit most from proactive PC consultations, with standardised criteria that are seamlessly integrated into routine workflows to ensure early identification without the need for individual provider opt-in. This approach is itself a true benefit to patients, their caregivers, and families. As health systems increasingly prioritise performance metrics, patient-centred care, and cost efficiency in care delivery, embedding value-based PC into ICU workflows is a necessary and achievable step forward.
Acknowledgement
We are grateful for the enormous contributions from the Mayo Clinic Kern Center.
Conflict of Interest
Patrick Wilson is a named inventor on a patent covering the algorithm discussed in this manuscript and receives royalties from its licensing through the Mayo Clinic.
References:
Adelson K, Paris J, Horton JR, Hernandez-Tellez L, Ricks D, Morrison RS, et al. Standardized criteria for PC consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017 May;13(5):e431–e440.
Bajwah S, Oluyase AO, Yi D, Gao W, Evans CJ, Grande G, et al. The effectiveness and cost-effectiveness of hospital-based specialist PC for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2020;(9).
Batzler YN, Schallenburger M, Schwartz J, Marazia C, Neukirchen M. The general public and young adults' knowledge and perception of PC: a systematic review. Healthcare (Basel). 2024 May 7;12(10):957.
Buang SNH, Loh SW, Mok YH, Lee JH, Chan YH. Palliative and critical care: their convergence in the pediatric intensive care unit. Front Pediatr. 2022 Jun 10;10:907268.
Carmichael H, Brackett H, Scott MC, Dines MM, Mather SE, Smith TM, et al. Early PC consultation in the burn unit: a quality improvement initiative to increase utilization. J Burn Care Res. 2021 Nov 24;42(6):1128–35.
Cox CE, Jones DM, Reagan W, Key MD, Chow V, McFarlin J, et al. Palliative care planner: a pilot study to evaluate acceptability and usability of an electronic health records system-integrated, needs-targeted app platform. Ann Am Thorac Soc. 2018 Jan;15(1):59–68.
Courtright KR, Srinivasan TL, Madden VL, Karlawish J, Szymanski S, Hill SH, et al. “I don’t have time to sit and talk with them”: hospitalists’ perspectives on PC consultation for patients with dementia. J Am Geriatr Soc. 2020 Oct;68(10):2365–72.
Effendy C, Yodang Y, Amalia S, Rochmawati E. Barriers and facilitators in providing PC in adult intensive care units: a scoping review. Acute Crit Care. 2022;37(4):516.
Flieger SP, Chui K, Koch-Weser S. Lack of awareness and common misconceptions about PC among adults: insights from a national survey. J Gen Intern Med. 2020 Jul;35(7):2059–64.
Hall A. Financial side effects: why patients should be informed of costs. Hastings Cent Rep. 2014;44:41–7.
Institute of Medicine. Dying in America: improving quality and honoring individual preferences near the end of life. Washington, DC: National Academies Press; 2015.
Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016 Nov 22;316(20):2104–14.
Kistler EA, Stevens E, Scott E, Philpotts LL, Greer JA, Greenwald JL. Triggered PC consults: a systematic review of interventions for hospitalized and emergency department patients. J Pain Symptom Manage. 2020 Aug;60(2):460–75.
May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015 Sep 1;33(25):2745–52.
McDarby M, Carpenter BD. Barriers and facilitators to effective inpatient PC consultations: a qualitative analysis of interviews with PC and non-PC providers. Am J Hosp Palliat Care. 2019;36(3):191–9.
Meddick‐Dyson SA, Boland JW, Pearson M, Greenley S, Gambe R, Budding JR, et al. Implementing PC in the intensive care unit: a systematic review and mapping of knowledge to the implementation research logic model. Intensive Care Med. 2024;50(11):1778–90.
Murphree DH, Wilson PM, Asai SW, Quest DJ, Lin Y, Mukherjee P, et al. Improving the delivery of PC through predictive modeling and healthcare informatics. J Am Med Inform Assoc. 2021 Jun;28(6):1065–73.
National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care. 4th ed. Richmond, VA: National Coalition for Hospice and Palliative Care; 2018.
Rao K, Goldstein NE, Peikes DN, Polt L, Kornitzer B. Effects of primary care-led, integrated PC for Medicare patients in a value-based model. J Pain Symptom Manage. 2024 Mar;67(3):195–203.
Romano AM, Gade KE, Nielsen G, Havard R, Harrison JH Jr, Barclay J, et al. Early PC reduces end-of-life intensive care unit (ICU) use but not ICU course in patients with advanced cancer. Oncologist. 2017 Mar;22(3):318–23.
Wilson PM, Ramar P, Philpot LM, Soleimani J, Ebbert JO, Storlie CB, et al. Effect of an artificial intelligence decision support tool on PC referral in hospitalized patients: a randomized clinical trial. J Pain Symptom Manage. 2023 Jul;66(1):24–32.
Wysham NG, Hua M, Hough CL, Gundel S, Docherty SL, Jones DM, et al. Improving ICU-based PC delivery: a multicenter, multidisciplinary survey of critical care clinician attitudes and beliefs. Crit Care Med. 2017 Apr;45(4):e372–8.

















