ICU Management & Practice, Volume 23 - Issue 3, 2023
The preanalytical phase of the blood gases study is the most susceptible to errors, causing increased time and costs for patients and hospitals. Knowledge and training of the involved health personnel must be constant to improve results.
Introduction
Arterial or venous blood gases test is a frequent tool in the Intensive Care Unit (ICU) commonly used for the respiratory system evaluation during respiratory failure and mechanical ventilation, the study of the acid-base status, or even macro- or micro-haemodynamic monitoring. However, it has been shown that up to 40% of blood gas analyses are in error during the procedure, and 4% of samples are analysed after 30 minutes of being obtained (Wooley et al. 2003). These errors can be ignored and go unnoticed, resulting in inadequate diagnoses and treatments (Davis et al. 2013). The study of blood gases can be divided into three phases of its analytical procedure: 1) the preanalytical phase, in which the supplies (syringe, heparin, antisepsis, etc.) are prepared, the sample is obtained and transported to the blood gas analyser; 2) the analytical phase, which corresponds to the blood gas analyser and includes the analytical processing itself, and 3) the post-analytical phase or interpretation of results by the clinician. Each phase presents errors, but they occur more frequently in the preanalytical phase, probably because it is the least automated and involves more personnel from different areas (Baird 2013). Blood gas analyses are an excellent tool for clinically evaluating critically ill patients, but the interpretation and decision-making based on erroneous results could be worse than not conducting them (Sánchez-Díaz et al. 2020).
Phases of the Analytical Procedure of Blood Gases Test
All the activity conducted in the laboratory is divided into three phases that are perfectly well-identified and delimited but closely related to each other. The analytical process of the blood gases study is divided into the following:
- Preanalytical phase
- Analytical phase
- Postanalytical phase
The majority of so-called laboratory errors usually occur outside the laboratory and are defined as “any defect from ordering tests to reporting and interpretation of results”. Of all the errors that occur in the analytical procedure of laboratory studies, up to 75% correspond to the preanalytical phase, 4% to the analytical phase, and 21% to the post-analytical phase (Kulkarni et al. 2020). The percentage difference of each phase is related to the number of manual or automated processes, the number and type of personnel involved, the external and internal quality controls, and the training to conduct all the processes (Sonntag 2009). Recently, through a measuring score applied to 54 undergraduate medical interns and first-year resident physicians, the knowledge of pre- and post-analytical phases of blood gases was assessed. It was documented that none of the participants had the level of knowledge necessary to solve various clinical situations (Ojeda Bello et al. 2020). On the other hand, laboratory errors, mainly in the preanalytical phase, increase the resources and costs necessary for hospital care, accounting for about 2% of the hospital’s total operating costs. In addition, the hours lost due to these errors are approximately 24,027 a year (Green 2013).
Preanalytical Phase
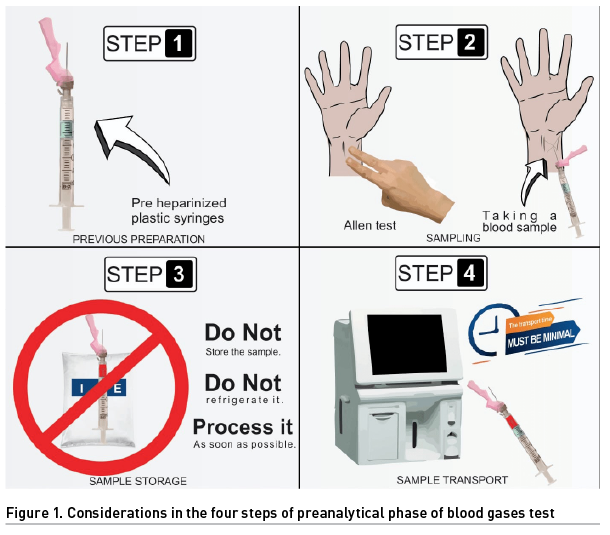
The preanalytical phase is characterised by having four steps, each one prone to various errors due to its apparent obviousness:
- Previous preparation
- Sampling
- Sample storage
- Sample transport
Step 1. Previous Preparation
It is the step where more mistakes are made and, therefore, where more could be avoided. The first one is due to the type of syringe used. The main difference between glass and plastic is the permeability (which is higher in plastic) to gases (oxygen and carbon dioxide) that increases with low temperature (Rodriguez Fraga et al. 2019). It is recommended to use pre-heparinised plastic syringes (dried calcium-balanced lithium heparin), which reduces the risk of sample dilution and chelation. In hospitals with limited resources, pre-heparinised syringes are not always available, so insulin syringes soaked in liquid sodium heparin are used. However, too much heparin can cause sample dilution (e.g., low haematocrit, no clinical correlation) and chelation (low ionised calcium, no clinical correlation), which translates into altered gasometric variables. Suspicion and lack of clinical correlation are always decisive in the assessment of blood gases. The recommendation is to use 8 to 12 IU or 0.012 to 0.04 ml of liquid sodium heparin per ml of blood. Consequently, in this context, this is impractical (WHO 2010). The implication of the needle diameter should not be overlooked since the smaller the diameter, the greater the haemolysis. This would be reflected in increased serum levels of potassium, magnesium, iron, etc. It is recommended to use needles of ≤ 25 gauge (G) or ≥ 0.5 mm diameter; these are inversely related, i.e., the smaller the gauge, the larger the diameter. Likewise, avoid puncturing a haematoma, let the alcohol used for antisepsis evaporate, avoid transferring the sample, or draw it from a blood test tube (Fang et al. 2008; Ogiso et al. 1983).
Step 2. Sampling
You can always consider a peripheral venous blood gas analysis, which is increasingly used. In this case, consider needles < 25 G in diameter, use a tourniquet for less than 60 seconds, let the antiseptic evaporate, draw the blood slowly, do not puncture through any haematoma, mix the sample gently, and preferably use syringes prefilled with dried calcium-balanced lithium heparin. The peripheral puncture can be obtained from any site, although it is preferred from the antecubital fossa. It is recommended to compress the puncture site after taking the sample for one minute (Kelly 2010; Schütz et al. 2019).
Regarding arterial blood sampling, initially assess collateral blood flow using the Allen test. The radial artery is the most used site for puncture due to its adequate collateral circulation through the ulnar artery. The same considerations as for peripheral venous blood gas analysis should be taken, except that there is no need for a tourniquet, and the compression of the puncture site will be for at least five minutes (WHO 2010).
Finally, the changes that occur in the variables measured in the blood gas analyser with respect to the amount of sample, from 3 to 1 ml, are not greater than 15%, so it is considered that the blood sample should not be less than 1 ml (Hedberg et al. 2009).
Step 3. Sample Storage
In this step, we start with the following key tips: do not store the sample, do not refrigerate it, and process it as soon as possible, preferably within 30 minutes (Montero Salinas et al. 2021). Remember that plastic syringes increase their permeability to gases (oxygen and carbon dioxide) at low temperatures, altering the values measured in the sample (Rodriguez Fraga et al. 2019). Another important and frequent problem is the presence of air bubbles inside the blood sample. Before the next step, they must be removed by homogenising the sample by rubbing gently and preferably within the first three minutes of obtaining the blood sample (Mohammadhoseini et al. 2015).
Step 4. Sample Transport
In a whole blood sample, aerobic metabolism can be maintained for a period of 15 to 30 minutes, after which the amount of oxygen and glucose will be depleted, altering the results. The transport time must be minimal so that these metabolic changes are minor. Transport can be manual (by health personnel) or automated (pneumatic tube). The first is the most used and acceptable. The second increases the incidence of haemolysis and the levels of oxygen partial pressure. The difference lies in the fact that manual transport has a universal gravitational constant (G) of 2G and the pneumatic tube transport of 15G, favouring these changes (Baird 2013). Approximately 40 to 80 ml of blood are used daily for diagnostic purposes, equivalent to one unit of packed red blood cells every 7 to 10 days (López et al. 2018).
The study of blood gases continues to be one of the most requested diagnostic studies in hospitals. Therefore, it is highly necessary that all health personnel involved master perfectly the four steps of the preanalytical phase to guarantee that the patient receives appropriate diagnoses and treatments (Baird 2013) (Figure 1). Although this procedure is considered safe with minimal risk, understanding the preanalytical phase would minimise complications such as unnecessary pain, bruising, vessel puncture site thrombosis, vascular or nerve injury, and infections (Castro 2022).
Conclusion
The preanalytical phase of the blood gases study is the most susceptible to errors, causing increased time and costs for patients and hospitals. These errors cause discomfort, complications, wrong diagnoses, or wrong therapeutic actions in patients. Knowledge and training of the involved health personnel must be constant to improve results. It should always be considered if a blood gas analysis is really necessary, so it is not performed routinely. Clinical suspicion is essential to detect errors; if the sample does not match the clinical characteristics of the patient, we advise you to obtain a new and better sample before making any decision.
Conflict of Interest
None.
References:
Baird G (2013) Preanalytical considerations in blood gases analysis. Biochem Med (Zagreb). 23:19-27.
Castro D, Patil SM, Keenaghan M (2022) Arterial Blood Gas. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Davis MS, Walsh BK, Sitting SE, Restrepo RD (2013) AARC clinical practice guideline: blood gas analysis and hemoximetry: 2013. Respir Care. 1694-1703.
Fang L, Fang SH, Chung YH, Chien ST (2008) Collecting factors related to the haemolysis of blood specimens. J Clin Nurs. 17(17):2343-51.
Green SF (2013) The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 46(13-14):1175-9.
Hedberg P, Majava A, Kiviluoma K, Ohtonen P (2009) Potential preanalytical errors in whole-blood analysis: effect of syringe sample volume on blood gas, electrolyte and lactate values. Scand J Clin Lab Invest. 69(5):585-91.
Kelly AM (2010) Review article: Can venous blood gas analysis replace arterial in emergency medical care. Emerg Med Australas. 22(6):493-8.
Kulkarni S, Piraino D, Strauss R et al. (2020) The Cost of Preanalytical Errors in INR Testing at a Tertiary-Care Hospital Laboratory: Potential for Significant Cost Savings. Laboratory Medicine. 51(3):320–324.
López A, García B, Gómez A et al. (2019) Concordance of the ions and GAP anion obtained by gasometry vs standard laboratory in critical care. Med Intensiva (Engl Ed). 43(9):521-527.
Mohammadhoseini E, Safavi E, Seifi S et al. (2015) Effect of Sample Storage Temperature and Time Delay on Blood Gases, Bicarbonate and pH in Human Arterial Blood Samples. Iran Red Crescent Med J. 17(3):e13577.
Montero Salinas A, Pérez Ramos M, Toba Alonso F et al. (2021) Analysis of Arterial Blood Gas Values Based on Storage Time Since Sampling: An Observational Study. Nurs Rep. 11(3):517-521.
Ogiso T, Iwaki M, Yamamoto M (1983) Hemolysis induced by benzyl alcohol and effect of the alcohol on erythrocyte membrane. Chem Pharm Bull (Tokyo). 31(7):2404-15.
Ojeda Bello JA, Cruz López C, Menéndez Acuna EP et al. (2020) Conocimiento de la fase preanalítica y postanalítica de la gasometría arterial en médicos residentes e internos. Educ Med.
Rodríguez Fraga O, Navarro Segarra X, Galán Ortega A et al. (2019) Recomendaciones preanalíticas para la medición del equilibrio ácido-base y los gases en sangre. Rev Lab Clin 12(4):e66-e74.
Sánchez Díaz JS, Peniche Moguel KG, Rivera Solís G et al. (2020) Hemodynamic monitoring with two blood gases: a tool that does not go out of style. Colomb. J. Anesthesiol. 49(1).
Schütz N, Roth D, Schwameis M et al. (2019) Can Venous Blood Gas Be Used as an Alternative to Arterial Blood Gas in Intubated Patients at Admission to the Emergency Department? A Retrospective Study. Open Access Emerg Med. 11:305-312.
Sonntag O (2009) Analytical interferences and analytical quality. Clin Chim Acta. 404(1):37-40.
Woolley A, Hickling K (2003) Errors in measuring blood gases in the intensive care unit: effect of delay in estimation. J Crit Care. 18(1):31-7.
WHO (2002) Use of anticoagulants in diagnostic laboratory investigations, WHO/DIL/LAB/99.1 Rev 2.
WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. Geneva: World Health Organization (2010) 5, Arterial blood sampling. Available at https://www.ncbi.nlm.nih.gov/books/NBK138661/











