ICU Management & Practice, ICU Volume 15 - Issue 3 - 2015
Over the last decades the number of immunocompromised patients has increased in parallel with improvements in transplantation science and alongside the development of numerous new classes of immunosuppressive agents offering novel therapy for a wide range of diseases. For example, an estimated 114,690 solid organ transplants were performed globally in 2012 (Global Observatory on Donation & Transplantation 2014). This growing population, however, also represents an increasing number of at-risk hosts. Of great concern, the incidence of serious infections and severe sepsis has clearly increased over time (Martin et. al. 2003).
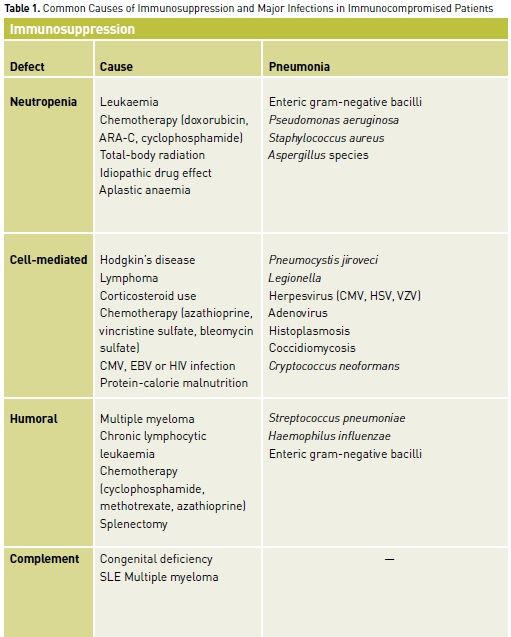
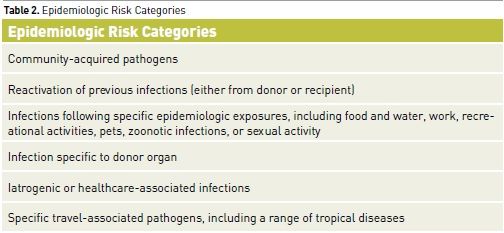
For clinicians and their patients, recognition of the immunocompromised state is imperative. Infections in such patients can involve unusual organisms requiring atypical pharmacological therapy. In addition to having increased susceptibility to common community-acquired and nosocomial pathogens, immunocompromised patients are vulnerable to opportunistic pathogens (e.g., Cryptococcus, Candida, and Aspergillus species) and to reactivation of endogenous but latent organisms (e.g., Herpesviruses, Toxoplasma gondii, Pneumocystis jiroveci) (Tables 1 and 2). Infection in immunocompromised patients with these pathogens can present with minimal signs and symptoms or with atypical features in unusual locations. This can considerably delay the diagnosis if the presence of immunocompromise is not appreciated. Although the risk of mortality is high in these patients given the underlying immunosuppression and the unusual nature of the involved organisms, outcome can be optimised by early diagnosis and aggressive treatments with specific pharmacotherapy.
An understanding of
terminology is important. An immunocompromised patient is one in whom any
aspect of host defence is deficient. In contrast, immunosuppression occurs when
immune defences are specifically impaired. The approach to infections in the
immunocompromised patient can be straightforward even though the variety of
infections that can be encountered is quite broad. First, the likelihood of a
given opportunistic infection is typically related to the nature, severity and duration
of the immune deficit. Finally, the duration of the immune deficit also helps
define the probable infecting agents.
A basic understanding of the elements of host defence and immunity is required to appreciate the likely cause of infection in any given immunocompromised patient. Both specific (immune) and nonspecific (nonimmune) host defences exist. Nonspecific defence elements include intact integumentary barriers. Defects of the integument such as seen in burns, severe eczema, or some forms of chemotherapy effectively denude the body of its primary defence against the normal microbial milieu. Similarly, invasive intravascular catheters, intubation, trauma and operative procedures disrupt the normal barriers to microorganisms.
Specific immune defects can be categorised into 4 clinically relevant groups. The first involves polymorphonuclear leukocytes (PMNs), which are responsible for phagocytosis and killing of extracellular microbes. The most common defect of PMN function is related to their absence. The second broad group of immune defects involves cellmediated immunity. This term encompasses processes by which intracellular pathogens as well as malignant and virus-infected cells are eliminated. Cell-mediated immunity involves monocytes/macrophages and T-lymphocytes. The third clinically relevant category of immune defects involves the humoral arm of the immune system. Humoral immune function involves B-lymphocytes that clonally proliferate to produce appropriate specific antibodies to foreign antigens. Anatomical or functional asplenia results in an immune defect similar to that seen with humoral deficits. The final category of immune defects involves the complement cascade, which is one of the major amplification pathways of the normal immune response.
Infections In Neutropenic Patients
Most patients become neutropenic as a consequence of leukaemia, the treatment of malignancy with chemotherapy, bone marrow transplant, or occasionally due to aplastic anaemia or idiosyncratic drug reactions. Although overt focal processes may occur, fever is the typical presenting feature in most patients with infection and severe neutropenia (<100 PMN/μL). Because of the frequent absence of focal findings, neutropenic patients with fever (>38.3°C) in the absence of a defined infection site must be assumed to have infection. When infection is identified it is usually found in the periodontium, oropharynx, lung, distal oesophagus, colon, perianal area or skin. Bacterial and fungal pathogens dominate in all patients with neutropenia regardless of its cause. Patients with neutropenia secondary to untreated leukaemia/lymphoma or aplastic anaemia are also at increased risk of reactivation of herpes viruses resulting in severe HSV mucositis, disseminated varicella-zoster, and cytomegalovirus (CMV) infection.
The dominant predictors of risk of infection during neutropenia due to chemotherapy are the degree and duration of neutropenia (Klastersky et. al. 1988). Neutropenia due to drug reactions, aplastic anaemia or congenital cyclic neutropenia involves a relatively isolated immune defect without mucosal injury. In contrast, cytotoxic chemotherapy impairs mucosal integrity and phagocytic function of surviving neutrophils and also adversely affects both humoral and cellmediated immunity. When fever and infection occur in the first few days (<1 week) of neutropenia, gram-positive cocci are frequently responsible. In those with more prolonged neutropenia, gram-negative bacilli become problematic in the second and third weeks. After 3 weeks of neutropenia, there is an increase in the incidence of opportunistic fungal infections, particularly with Candida and Aspergillus species and more exotic pathogens such as Mucor species, Trichosporon species and even Fusarium species.
Typically, infections in patients with neutropenia are due to endogenous bacteria (although half may be hospital-acquired). Nosocomial acquisition may have occurred from physical contact (gram-negative rods, gram-positive cocci), water sources (Legionella) or air (Aspergillus). Potential sources of infection with endogenous organisms include skin (e.g., Staphylococcus species, coagulase negative Staphylcoccus species, Corynebacterium species, Bacillus species, gram-negative rods, and Candida) and gut (e.g., gram-negative rods [Escherichia coli,Klebsiella species, and Pseudomonas aeruginosa] and Candida species). In recent decades, organisms such as Stenotrophomonas maltophilia causing pneumonia, Burkholderia cepacia causing line sepsis and Aeromonas hydrophila leading to necrotising fasciitis, Leuconostoc species, Capnocytophaga species and Rhodococcus equi have all emerged as important pathogens. This shift has occurred for a variety of reasons, including use of prophylactic antibiotics and chemotherapeutic regimens causing greater mucositis. A number of principles, developed in the 1960s, continue to be relevant to the management of fever from an undefined source in the neutropenic host. First, neutropenic patients (PMN count <500 cells/μL) with fever (temperature >38.5°C on one occasion or >38°C on two occasions) should be started on empirical antibiotic therapy (Freifeld et. al. 2011). Second, as a rule, broad empirical antibiotics should be used, particularly in the ICU. Third, broad-spectrum antibiotics should be continued for the duration of the neutropenia or for 10 to 14 days if the absolute neutrophil count (ANC) recovers to greater than 500/μL (whichever is longer). Fourth, the specific choice of the initial antibiotic regimen is dependent on the microbial flora of the local environment. Finally, it is a well-accepted principle of therapy that if fever persists or recurs in a neutropenic patient after 4 to 7 days of broad-spectrum antibacterial therapy, empirical antifungal therapy is required.
Infections in Solid Organ Transplantation Patients
Since 1980 solid organ transplantation success rates have increased dramatically. This improvement has largely been due to the introduction of more potent but selective immunosuppressive compounds as well as advances in surgical technique. Immunosuppression in these patients primarily reflects iatrogenic pharmacologically-induced depression of cell-mediated immunity (cellular immune function) for purposes of graft retention. Pharmaceutical agents that induce defects of cell-mediated immunity include high-dose steroids (>60 mg/day prednisone equivalent), azathioprine, lowdose cyclophosphamide, vincristine, bleomycin, muromonab-CD3), antilymphocyte or thymocyte globulin, and, to a lesser extent, cyclosporine and tacrolimus.
Post-transplantation infections are divided into 3 major categories based on the postsurgical time period (Fishman 2007; de Pauw and Rubin 2007; Rubin 2002; Rubin et. al. 1981). In the first period, the first month post-transplant, the majority of infections are similar to those in any postsurgical patient. In the second time period, the period from the second until the sixth month post-transplant, opportunistic infections predominate. The third time period, 6 months or more post-transplant, is characterised by infections similar to those in an immunocompetent individual. However, a continuing requirement for high dose pharmacological immunosuppression or the presence of graft versus host disease will substantially alter these timelines.
Most early postoperative infections in solid organ transplant recipients are similar to those occurring in immunocompetent patients; however, their clinical course may be much more severe. Surgical wound and IV catheter infections, urinary tract infections, and pneumonia are typical. Pathogens responsible for these infections are typically nosocomial in origin and will carry resistance patterns endemic to ICU organisms. The majority of remaining early post-transplant infections are caused by reactivation of latent or subclinical infections that were present in the recipient before transplantation. Reactivation is triggered by perioperative nonspecific insults and intense immunosuppression. Typical organisms include HSV, Mycobacterium tuberculosis, geographically restricted mycoses (Histoplasma capsulatum and Coccidioides immitis) and, occasionally, Strongyloides stercoralis and Toxoplasmosis gondii. Opportunistic pathogens do not normally present in this early postoperative period, since they require a prolonged period of immunosuppression to manifest.
Following the first month post-transplantation, defects of cellular immunity due to pharmacological intervention begin to have a greater impact on the nature of infections. The risk for infections is maximal between 1 and 6 months (with serious life-threatening infections occurring at 3 to 4 months after transplantation). The immunomodulating viruses (CMV, Epstein-Barr virus (EBV) and human immunodeficiency virus (HIV)) are one of the major infectious concerns during this period. Infection with these viruses can further increase the patient’s risk of developing opportunistic infections caused by Pneumocystis jiroveci, Listeria monocytogenes, Aspergillus, Nocardia and Cryptococcus species.
Normally, at 6 months post-transplantation pharmacological immunosuppression is minimised and graft function is optimal. At this point most infections in graft recipients are similar to those of immunocompetent individuals. The actual organ transplanted is crucial to determining the risk of infection especially in the first three months. For example, lung transplant recipients tend to exhibit recurrent pneumonias, involving both standard bacterial pathogens and opportunistic organisms. Liver transplant recipients exhibit biliary sepsis with increased frequency. Renal transplants are often complicated by recurrent urinary tract infection.
Infections in Haematologic Stem Cell Transplantation Patients
Three broad time periods corresponding to the nature of infectious risk have been defined for bone marrow recipients. The first encompasses the pre-engraftment period occurring from bone marrow ablation until 30 days post-transplant. The second time period, the post-engraftment period lasts from 30 days to 100 days post-transplant. The third time period, the late post-trans plantation period, begins 100 days post-transplant (Rubin et. al. 1981; Stamm et. al. 1982; Tolkoff and Rubin 2004; Keiser and Nutman 2004; Barnes and Stallard 2001; Bowden and Meyers 1994; Sable and Donowitz 1994).
In the first time period, infections are primarily related to the severe neutropenia and mucositis caused by the cytotoxic conditioning regimen given for the transplant. Clinical disease and treatment are therefore similar to those for other febrile neutropenic patients. Various prophylactic regimens to prevent infectious complications may be given in this period. These can influence predominant pathogens, shifting bacterial species to more resistant gram negatives, and increasing the incidence of colonisation with fluconazole-resistant Candida species. The most common pathogens in this time period include HSV and human metapneumovirus.
The second time period, 30 days to 100 days post-transplant includes infection risk due to antirejection immunosuppressive regimen leading to depressed cell-mediated immunity. However, immune dysfunction caused by the bone marrow ablating conditioning regimen as well as graft versus host disease, may contribute to the increased infection risk during this period. Since allogeneic transplants require greater immunosuppression, they tend to have higher rates of infection than autologous transplants. Because cell-mediated immunity is predominantly affected, infections in this period involve pathogens similar to those seen in organ transplant recipients. The dominant concerns are CMV, herpes simplex virus (HSV) and varicella zoster virus (VZV) (usually reactivation), Pneumocystis jiroveci, invasive Aspergillus, Candida species, rarely HHV-6 and Cryptosporidum.
During the third time period, after 100 days post-transplant, VZV reactivation and viral respiratory infections become more common. About 40% of patients will develop a significant VZV infection (either zoster or varicellalike syndrome) during their post-transplant course, and one third will experience disseminated disease associated with high mortality. Potential causes of viral respiratory tract infections in the late post-transplant period include respiratory syncytial virus and parainfluenza. They are the most common causes of viral pneumonia in this population and carry a high mortality rate.
Infections in Patients Receiving Therapy with Biologics
Three groups of biological interventions exist for control of systemic inflammatory diseases. These groups:
1. interfere with cytokine function;
2. inhibit T-cell activation, and 3. deplete B-cells.
The first group contains the current tumour necrosis factor (TNF)-α blocker agents (etanercept, infliximab, adalimumab, certolizumab, golimumab, and natalizumab); the interleukin-1 inhibitors (anakinra); and the interleukin-6 inhibitors (tocilizumab and sirukumab). In the second group, abatacept and belatacept inhibits T-cell activation by preventing T-cell receptors from binding to costimulatory molecules. Rituximab, an anti- CD20 antibody, depletes B cells as a member of the third group.
Data from several large registries consistently have found TNF-α inhibitors to be associated with an increased risk for serious infections, with nearly a 5-fold relative increase in the rate of infections in the first 90 days after starting therapy compared with controls (Bongartz et. al. 2006; Galloway et. al. 2011; Crawford and Curtis 2008). Important bacterial infections include tuberculous and nontuberculous mycobacterial infections; Listeriosis, Legionellosis, and Nocardiosis (Kuehn 2011).
Viral infections such as acute or chronic hepatitis B and C are contraindications to therapy with TNF-α inhibitors because of the risk of reactivation. Reactivation of varicella zoster and CMV is also known to occur. Fungal infections due to Histoplasmosis, Coccidioidomycosis, Aspergillus, Rhizopus, and Cryptococcus have all been documented. Rituximab has been associated with Pneumocystis jiroveci and Aspergillosis.
Conclusion
Immunocompromised patients are vulnerable to an exceptionally broad variety of infections. A methodologic assessment of a patient’s risk for infection, described above, should assist clinicians in adeptly defining targeted diagnostic testing and therapeutic interventions.
References:
Barnes RA, Stallard N (2001) Severe infections after bone marrow transplantation. Curr Opin Crit Care, 7(5): 362-6.
Bongartz T, Sutton AJ, Sweeting MJ et al. (2006) Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA, 295(19): 2275-85.
Bowden RA, Meyers JD (1994) Infection complicating bone marrow transplantation. In: Rubin RH, Young LS, eds. Clinical approach to infection in the compromised host. 3rd ed. New York: Plenum, pp. 601-28.
Crawford M, Curtis JR (2008) Tumor necrosis factor inhibitors and infection complications. Curr Rheumatol Rep, 10(5): 383-9.
de Pauw B, Rubin RH (2007) Principles of antimicrobial therapy in the transplant recipient, Transplant Infect Dis, 9(1): 1-2.
Fishman JA (2007) Infection in solidorgan transplant recipients. N Engl J Med, 357(20): 2601-14.
Freifeld AG, Bow EJ, Sepkowitz KA et al. (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis, 52(4): e56-93.
Galloway JB, Hyrich KL, Mercer LK et al. (2011) Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology, 50(1): 124-31.
Global Observatory on Donation & Transplantation (2014) 2012 activity data. [Accessed: 10 September 2015] Available from http://www.transplant-observatory. org/Pages/Data-Reports.aspx
Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev, 17(1): 208-17.
Klastersky J, Zinner SH, Kuehn BM (2011) Growing list of infections linked to TNF blockers. JAMA, 306(13): 1430.
Martin GS, Mannino DM, Eaton S et al. (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med, 348(16): 1546-54.
Rubin RH (2002) The direct and indirect effects of infection in liver transplantation: pathogenesis, impact, and clinical management. Curr Clin Topics Infect Dis, 22(22): 125-54.
Rubin RH, Wolfson JS, Cosimi AB et al. (1981) Infection in the renal transplant recipient. Am J Med, 70(2): 405-11.
Sable CA, Donowitz GR (1994) Infections in bone marrow transplant recipients. Clin Infect Dis, 18(3): 273-81.
Stamm AM, Dismukes WE, Simmons BP et al. (1982) Listeriosis in renal transplant recipients: report of an outbreak and review of 102 cases. Rev Infect Dis, 4(3): 665-82.
Tolkoff-Rubin NE, Rubin RH (2004) Infection in organ transplant recipients. In: Gorbach SL, Bartlett JB, Backnow NR, eds. Infectious diseases, 3rd ed., Philadelphia: Lippincott Williams and Wilkins, pp. 1111-23.