ICU Management & Practice, Volume 23 - Issue 2, 2023
Extracorporeal membrane oxygenation is a resource that is accessible in hospitals and intensive care units all over the world. In serious situations, ECMO therapy is intended to provide haemodynamic and/or ventilatory support. Because of this, many people refer to the ECMO patient as "the most critical patient." As a result, there is a very high likelihood that a critical disease will result in physical disabilities. A well-timed commencement to overcome such problems is crucial, as is a rehabilitation team that is well-trained and experienced.
Introduction
The critically ill patient is at high risk of developing functional alterations derived from the severity of the disease. The usage of drugs needed in critical illness, prolonged immobility and other factors directly impact muscle health (Martínez-Camacho et al. 2020). The muscle is an organ that can be severely affected in the intensive care unit (ICU) in various ways, such as fatty infiltrates, necrosis, ion channel alterations, mitochondria involvement and denervation. Together they manifest as generalised muscle weakness, usually symmetrical and that has no other explanation apart from critical pathology. This is known as ICU-acquired weakness (ICUAW) (Calixto-Mejía et al. 2020).
Currently, with the advancement in technology, life support measures have increased the therapeutic arsenal for critically ill patients and extracorporeal assistance is a reality in most units around the world. Among these devices, extracorporeal membrane oxygenation (ECMO) stands out, which, depending on the configuration, can provide pulmonary support (VV-ECMO) or haemodynamic support (VA-ECMO)(Combes et al. 2020; Shah et al. 2021). One of the most used indications for VV-ECMO is severe acute respiratory distress syndrome (ARDS) that does not respond to conventional management (protective mechanical ventilation, prone position, and neuromuscular blockade). An adequate selection of ECMO candidate patients is crucial to obtain favourable results.
ECMO and Physiotherapy
Before connecting the patient to ECMO, a management plan must be established, and the purpose of extracorporeal therapy must be determined. Nevertheless, ECMO can work as a bridge-to-resolution, bridge-to-transplantation or gain some time while the problem that conditioned the patient to require assistance can be resolved. Given the characteristics of the patients, it is possible to keep them hospitalised for a prolonged period in the ICU (Hayes et al. 2021). This puts these patients at a higher risk of developing relevant functional sequelae, post-intensive care syndrome, difficulty in weaning from mechanical ventilation, etc. Early mobilisation (EM) is a widely used strategy for the prevention of ICU-acquired weakness (ICUAW) in critically ill patients, and patients undergoing ECMO therapy are no exception.
Studies currently support the safety of the implementation of EM in this group of patients, with low adverse effects and many functional benefits (Hogdson et al. 2014; Cameron et al. 2015; Hayes et al. 2021). To carry out an EM programme, it is necessary to implement a highly trained rehabilitation team due to the special characteristics of this group of patients.
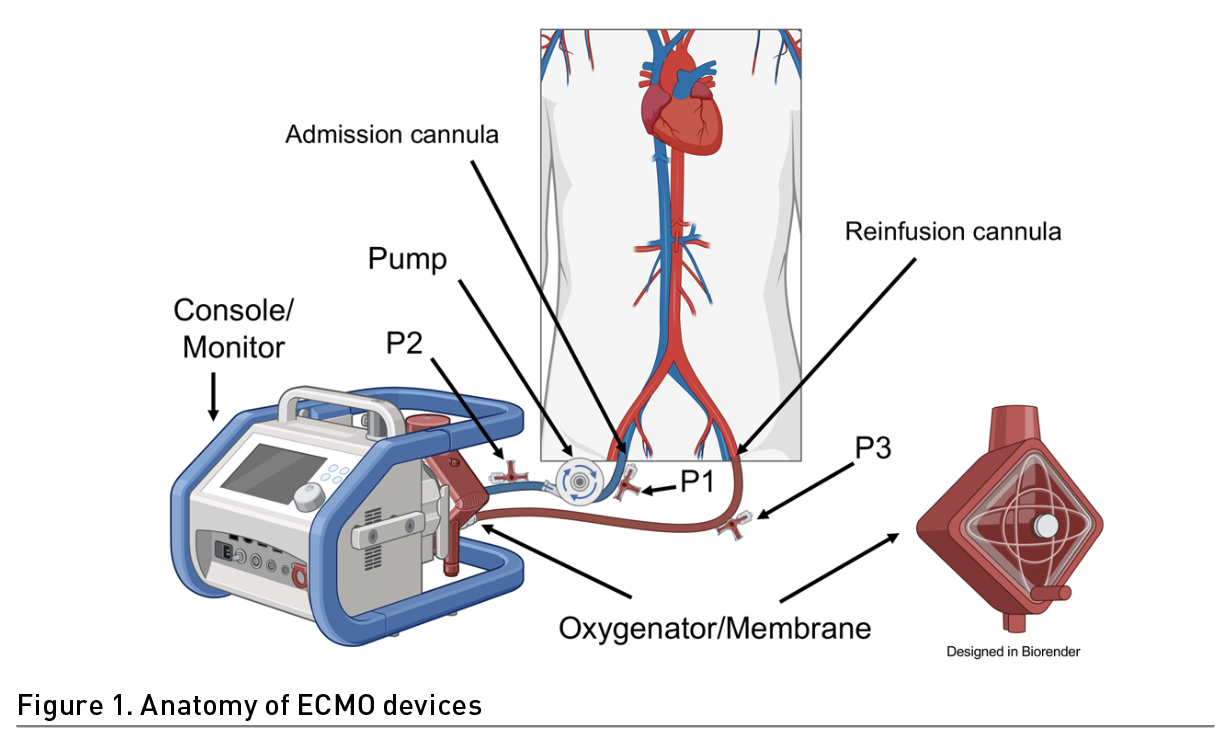
Identifying several situations in patient care before carrying out the exercise in patients on ECMO is crucial. For instance, the ECMO configuration, the type of support provided and the type of cannulation (Table 1). In addition, it is necessary to inspect the extraction and drainage cannula visually (Wieruszewski et al. 2023; Combes et al. 2020) (Figure 1). This can alert us to a malfunction of the membrane. The normal colouration in the extraction cannula is dark red and in the return cannula bright red. This is due to the blood characteristics (deoxygenated and oxygen-rich blood respectively). Also, the inspection of the cannula entry site to identify bleeding, displacement, or infection is crucial for the prevention of adverse effects (Mossadegh et al. 2016).
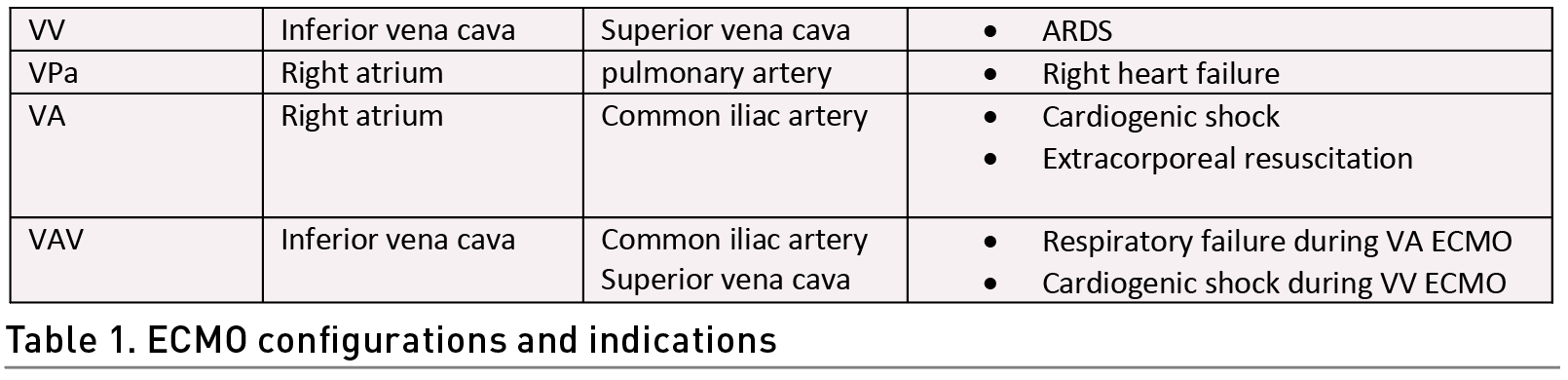
Special care must be taken when moving the cannulas, especially in the lower limbs, since they can be bent and thus increase resistance, in addition to reducing the extraction flow; this is prevented by avoiding hip flexion >90° (Raurell-Torredà et al. 2021). It is recommended to fix the cannulas during the mobilisation of the patient to avoid any adverse event.
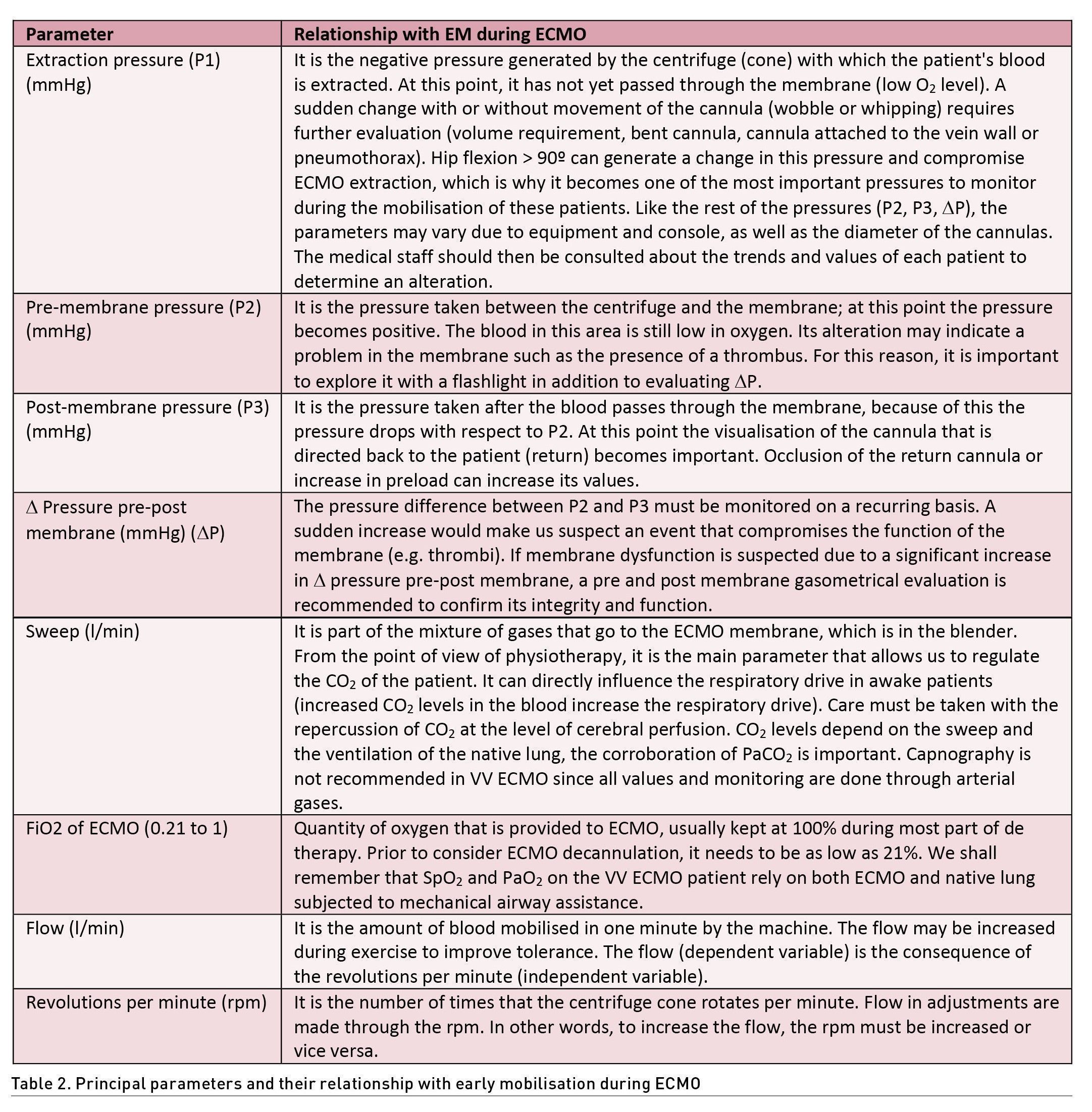
Within the general monitoring of the patient on ECMO, a DO2/VO2 ratio of at least 3:1 must be guaranteed to consider clinical stability (Lorusso et al. 2021). For this reason, it is essential to maintain SpO2 >90% and SvO2 >60%, which may have a direct correlation. On the other hand, a haemoglobin level >10 g/d is recommended; however, in some cases values >8g/dL, fibrinogen >150 mg/dL and platelets >80,000/mcL may be considered. If available, rotational thromboelastography can help in haematological monitoring. Haemodynamic stability must be continuously monitored at the patient's bedside, verifying perfusion windows, blood pressure, echocardiography and, if required, advanced invasive monitoring. The measurement of cardiac output is important for the termination of the flow and the revolutions per minute (rpm) in ECMO VV, recommending that it be close to 80% or 60 ml/Kg/Hr (Tonna et al. 2021; Ki et al. 2019; Shekar et al. 2020). It should be taken into account that exercise, especially active exercise, can increase oxygen consumption and the need for increased cardiac output, so it may be necessary to modify some parameters such as rpm, flow, FiO2 or sweep gas, as well as the monitoring of ECMO pressures, which in the face of their alteration, rehabilitation should be temporarily suspended until resolution (Table 2) (Mossadegh et al. 2016).
Considerations to Mobilise VV ECMO Patients
Currently, the main indication for VV ECMO is severe ARDS, which entails some specific challenges for mobilisation in this population (Abrams et al. 2014; Pruijsten et al. 2014; Haji et al. 2021) These patients usually have significant lung parenchyma damage or inflammation that requires specific measures such as ultraprotective mechanical ventilation, neuromuscular blockade, and deep sedation. One of the most complex situations in the rehabilitation of a patient with ARDS in ECMO is to determine when they are ready for functional progression.
One of the main limitations is the need for deep sedation and neuromuscular blockade. There are some cases where it is necessary to combine ECMO with prone ventilation to reach oxygenation goals (Combes et al. 2020; Tonna et al. 2021). Oxygenation depends mainly on the membrane and the native lung; the latter being severely affected. As in most critically ill patients, sedation withdrawal is recommended as soon as possible to minimise deleterious effects. Early tracheostomy (as in the first seven days after intubation) can help in the rehabilitation process for better airway management, and a higher level of mobility, in addition to reducing swallowing and phonation complications derived from prolonged intubation (Nukiwa et al. 2022; DiChiacchio et al. 2020; Shaw et al. 2015).
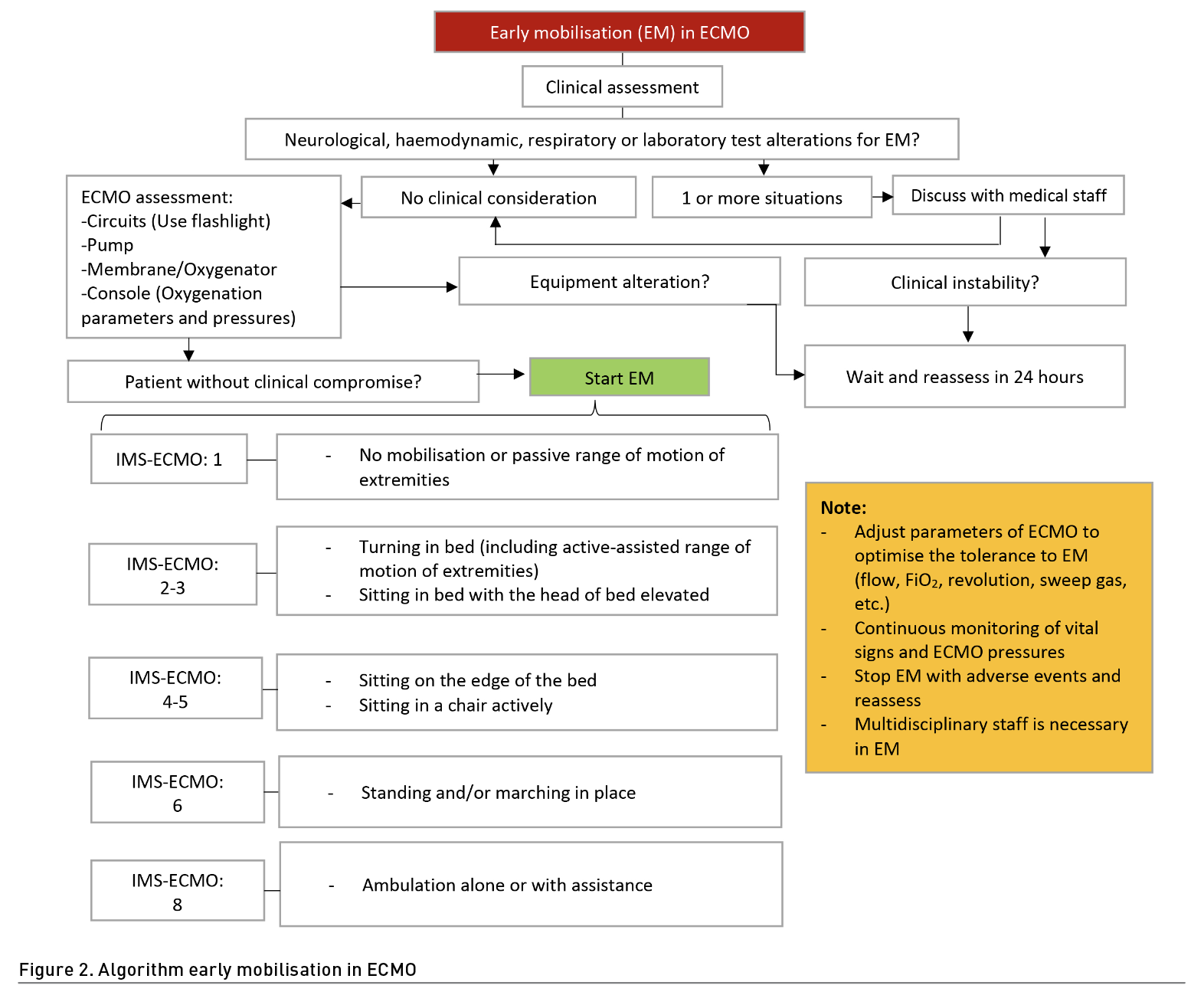
Thus far, there is no evidence to mobilise patients in prone position; clinical stability will be the main priority in decision-making (Hogdson et al. 2014; Gómez et al. 2022). The approach can begin with passive interventions such as passive mobilisation, positioning, stretching, and neuromuscular electrostimulation (NMES) to maintain range of motion, joint health, and muscle preservation; however, priority should always be given to the extent of those possible from active muscle contraction and resisted exercise in and out of bed. Verticalisation can be useful starting with sitting in bed and continuing at the edge of it, depending on haemodynamic stability. Later, when the patient can overcome gravity with the lower extremities and the ability to support the trunk, standing or walking can be considered. If the patient is not cooperative, an alternative can be using hoists, cranes or verticalisation tables. All interventions should be aimed at improving functionality and activities of daily living (Haji et al. 2021). It is extremely important to establish therapeutic objectives based on functional scales applied in ECMO such as the ICU-Mobility Scale (IMS) or its adaptation for patients with ECMO (IMS-ECMO) (Abrams et al. 2021) (Figure 2).
One of the main concerns with physical exercise in patients with ARDS, especially on ECMO, is the possibility of generating patient self-inflicted lung injury (PSILI). So far, there is no evidence of the impact of exercise on lung injury in this population (Langer et al. 2016). As a precautionary measure, some parameters such as P0.1, muscle pressure (Pmus), muscle pressure index (MPI) or dynamic transpulmonary driving pressure (∆PL) can be evaluated to monitor mechanical ventilation in spontaneous modes, which is recommended in patients who are already awake and who are in an active rehabilitation programme (Pavez et al. 2022). On the other hand, continuous monitoring of tidal volume and respiratory rate could be sufficient during the rehabilitation session, in addition to respiratory and haemodynamic stability. As previously mentioned, it may be necessary to modify some of the ECMO parameters to improve exercise resistance; increasing pressure support or FiO2 on the ventilator can contribute to this objective.
The ECMO rehabilitation programme can be very extensive in these patients because ARDS tends to evolve slowly compared to other respiratory pathologies. In addition, in some cases the patient must be kept mechanically ventilated until a lung transplant is available, which can take considerable time. The importance of rehabilitation in critically ill patients has been demonstrated, as well as the patient undergoing ECMO therapy. Therefore, a well-trained team is needed to mobilise a severely ill patient such as those assisted through extracorporeal machines in order to increase the chances of recovery and improve its long-term prognosis.
Conflict of Interest
None.
References:
Abrams D, Javidfar J, Farrand E et al. (2014) Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Critical care. 18(1):R38.
Calixto AAM, Martínez NGM, Nieto ORP et al. (2018) Movilización temprana como prevención y tratamiento para la debilidad adquirida en la Unidad de Cuidados Intensivos en pacientes en ventilación mecánica. Experiencia en un hospital de segundo nivel. Eur Sci J. 14(21):19-30.
Cameron S, Ball I, Cepinskas G et al. (2015) Early mobilization in the critical care unit: A review of adult and pediatric literature. J Crit Care. 30(4):664- 672.
Combes A, Schmidt M, Hodgson CL et al. (2020) Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Medicine. 46(12):2464–2476.
DiChiacchio L, Boulos FM, Brigante F et al. (2020). Early tracheostomy after initiation of venovenous extracorporeal membrane oxygenation is associated with decreased duration of extracorporeal membrane oxygenation support. Perfusion. 35(6):509-514.
Gómez GA, Martínez CMA, Jones BRA et al. (2022) Ten Overlooked Mistakes During Early Mobilisation in the Intensive Care Unit. ICU Management & Practice. 22(3):146-149.
Haji JY, Mehra S, Doraiswamy P (2021) Awake ECMO and mobilizing patients on ECMO. Indian journal of thoracic and cardiovascular surgery. 37(Suppl 2):309-318.
Hayes K, Hodgson CL, Webb MJ et al. (2021) Rehabilitation of adult patients on extracorporeal membrane oxygenation: A scoping review. Australian critical care: official journal of the Confederation of Australian Critical Care Nurses, S1036-7314(21)00136-3.
Hodgson CL, Stiller K, Needham DM et al. (2014) Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 18(6):658.
Ki KK, Passmore MR, Chan CHH et al. (2019) Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensive care medicine experimental. 7(1):51.
Langer T, Santini A, Bottino N et al. (2016). Awake extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Critical Care. 20(1):150.
Lorusso R, Shekar K, MacLaren G et al. (2021) ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO Journal. 67(8):827-844.
Martínez CMA, Jones BRA, Gómez GA (2020) El fisioterapeuta en la Unidad de Cuidados Intensivos ¿un profesional necesario? Acta Med. 18(1):104-105.
Mossadegh C (2016) Monitoring the ECMO. Nursing Care and ECMO. 45-70.
Nukiwa R, Uchiyama A, Tanaka A et al. (2022) Timing of tracheostomy and patient outcomes in critically ill patients requiring extracorporeal membrane oxygenation: a single-center retrospective observational study. Journal of Intensive Care. 10(1):56.
Pavez N, Damiani LF (2022) Inspiratory and expiratory pause during pressure support ventilation: Maneuvers that we should incorporate into clinical practice. Medicina Intensiva. 46(4): 213-216.
Pruijsten R, van Thiel R, Hool S et al. (2014) Mobilization of patients on venovenous extracorporeal membrane oxygenation support using an ECMO helmet. Intensive Care Medicine. 40(10):1595-1597.
Raurell-Torredà M, Regaira-Martínez E, Planas-Pascual B et al. (2021) Early mobilisation algorithm for the critical patient. Expert recommendations. Enfermeria intensive. 32(3): 153–163.
Shah A, Dave S, Goerlich CE, Kaczorowski DJ (2021) Hybrid and parallel extracorporeal membrane oxygenation circuits. JTCVS techniques. 8:77-85.
Shaw JJ, Santry HP (2015) Who Gets Early Tracheostomy? Evidence of Unequal Treatment at 185 Academic Medical Centers. Chest. 148(5):1242-1250.
Shekar K, Buscher H, Brodie D (2020) Protocol-driven daily optimisation of venovenous extracorporeal membrane oxygenation blood flows: an alternate paradigm? Journal of thoracic disease. 12(11):6854–6860.
Tonna JE, Abrams D, Brodie D et al. (2021) Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO Journal. 67(6):601-610.
Wieruszewski PM, Ortoleva JP, Cormican DS, Seelhammer TG (2023) Extracorporeal Membrane Oxygenation in Acute Respiratory Failure. Pulmonary therapy. 9(1):109-126.












