ICU Management & Practice, ICU Volume 13 - Issue 1 - Spring 2013
Continuous glucose monitoring systems and therapeutic algorithms adapted to the actual insulin sensitivity are needed to prevent hypoglycaemia, hyperglycaemia and high glucose variability, all associated with poor outcome in intensive care unit (ICU) patients.
Introduction
A decade after the publication of the landmark Leuven I trial (Van
den Berghe et al. 2001), the issue of glucose control in the ICU is still a
matter of intense debate. After the initial enthusiasm inspired when authors of
the Leuven I trial reported a 40% relative decrease in mortality by tight
glucose control (TGC), several confirmatory trials failed to verify this benefit (Marik and Preiser 2010). A
major difference between the Leuven trials and other studies is that a much
lower amount of intravenous calories is given in other centres than Leuven. The
recent “Impact of Early Parenteral Nutrition Completing
Enteral Nutrition in Adult Critically Ill Patients” (EPaNIC) trial (Casaer et
al. 2011), which was performed in the same centre, demonstrated that
administering early parenteral nutrition (PN), as compared to late PN, when
enteral nutrition (EN) was not tolerated after one week, was in fact detrimental.
In theory, the effects of TGC by intensive insulin therapy should be
re-assessed when a lower caloric load is administered, in accordance with these
findings.
Not surprisingly, guidelines for glucose control (GC) swung from the recommendation of TGC to a more liberal threshold, i.e. a target range for blood glucose (BG) below 150 mg/dl (Jacobi et al. 2012) or even 180 mg/dl (Dellinger et al. 2013; Qaseem et al. 2011). In fact, a universally acceptable upper threshold for BG is probably elusive (Ichai and Preiser 2010) for several reasons, either related to the patients’ underlying condition (diabetes, neurological conditions or trauma, cardiac surgery) or to organisational aspects (nurse staffing, type and accuracy of glucose meter in use). Very interestingly, this decade of debates brought several advances to the field:
- Associations between each of the three domains of dysglycaemia—hypo, hyper, high variability—and poor outcome;
- Technological advances in the monitoring of blood glucose in ICU; and
- Development of automated, computer-assisted therapeutic insulin-algorithms.
Dysglycaemia Associations With Poor Outcome
Several investigators worldwide reported the associations between each of the three domains of dysglycemia (hypo, hyper, high variability) and poor outcome, with studies often involving a very large database (Badawi et al. 2012; Falciglia et al. 2009; Finfer et al. 2012; MacKenzie et al. 2011; Krinsley et al. 2013). At the very least, these very consistent findings indicate that each of these domains is a marker of the severity of disease. Iatrogenic components, such as insulin therapy, rapid correction of hypoglycaemia, steroids, and catecholamines, were also identified as causes of dysglycemia. The unsolved issue is now whether the prevention and avoidance of hypo, hyper and high glycemic variability is associated with an improved outcome. This key question is very difficult to answer when the achievement and maintenance of BG in a narrow range is precluded by the impossibility to continuously monitor BG. Intermittent BG measures can underestimate glycemic variability, and even episodes of severe hyperglycaemia or hyperglycemia can be missed (Joseph et al. 2009; Rice and Coursin 2012).A board ofexperts published quality criteria for the desirable performance of intermittent and continuous glucose meters to be used in an ICU (Finfer S, Wernerman J, Preiser JC, Cass T, Desaive T, Hovorka R, Joseph JI, Kosiborod M, Krinsley J, Mackenzie I, Mesotten D, Schulz M, Scott MG, Slingerland R, Van den Berghe G, Van Herpe T. Consensus Recommendations on "Measurement of Blood Glucose and Reporting Glycemic Control in Critically Ill Adults". Crit Care 2013 (in press)).
In relation to the unsolved key question, there are several investigational arguments supporting an improvement in outcome by maintaining BG within a narrow range (Ellger et al. 2006; Behrends et al. 2010) when faced with the potential role of stress hyperglycaemia, as this acts as a component of an adaptive mechanism to survive the initial post-injury phase (Soeters and Soeters 2012; Dungan et al. 2009). Therefore, the question will remain open until reliable means including continuous monitoring and adapted insulin algorithms to achieve and maintain blood glucose within a narrow range become available.
Continuous Glucose Monitoring
The question of whether outcome will be improved by maintaining BG
within a narrow range can only be answered when rapid, accurate,
interference-free, inert, and cost-effective continuous glucose monitoring
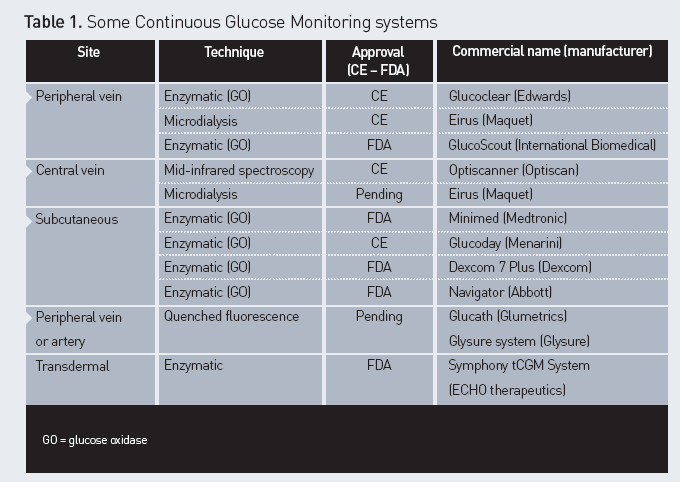
(CGM) systems are validated for clinical use (Rice and Coursin 2012). Several
techniques are proposed, as well as different sites of monitoring (Table 1).
However, technical improvements of these devices are still needed to achieve
optimal performance in ICU patients. The different problems reported are
related to interferences with drugs, or physico-chemical factors. The reliability
of a subcutaneous signal is limited in cases of poor skin perfusion, as in
shock, or in obese patients. The use of sensors connected to a peripheral
venous catheter can be limited by the quality of venous access. Unfortunately
the quality of the signal can hardly be measured and displayed on the screen of
these monitoring systems.
An option suggested in case of suboptimal signal is to
consider CGM as a warning system, designed to describe a trend of BG rather
than actual BG values. In such case, any BG value out of range should be
checked on an intermittent glucose meter.
Responding to the numerous limiting factors for the use
of continuous or nearcontinuous monitoring systems in ICUs, manufacturers are
working hard to improve the reliability of devices in the very complex and
changing environment. In particular, filters for potential chemical
interferents (i.e. drugs used in ICU patients) have been incorporated into
several devices. The lifespan of some of the disposable equipment has been
prolonged over a 24-hour period and time intervals between calibrations have
been increased, etc. Nevertheless, most of these devices have not yet been
validated for use in a heterogeneous population of medical and surgical ICU
patients over relevant time periods. The results of some of the ongoing validation
trials should be released soon.
Regarding the therapeutic impact of inaccuracies in the reading of BG
concentrations, error grids adapted to the actual insulin algorithms that are
in use in ICUs are needed. Indeed, the current Clarke Error Grid was developed
to assess the performance of intermittent glucose meters in diabetics, but it
is not adapted for use in the ICU. In particular, error grids adapted to the
actual target range and insulin algorithms have been published (Ellmerer
et al. 2006). Error grids for CGM have also been proposed (Kovatchev et al.
2004) and used to as-sess the impact of inaccuracies in the ICU (Brunner et al.
2011). A revision of the criteria required for clearance by the regulatory authorities
is currently underway.
The Future
When reliable CGM is available, the next advance will be the incorporation of an insulin algorithm that is adapted to actual insulin sensitivity and re-calculated after each BG reading. The current glucose intake and rate of insulin are needed for the calculation of insulin sensitivity, which largely varies in critically ill patients (Pretty et al. 2012). The validation of these individualised insulin algorithms can be performed in patients or in virtual cohorts of patients by physiological modelling (Chase et al. 2011; Pielmeier et al. 2010). It seems that computer-assisted insulin delivery systems will be a useful tool in the near future.
Conclusion
In spite of the current uncertainties regarding the optimal BG
levels to be achieved in critically ill patients, CGM systems and
individualised insulin algorithms are promising tools that will enable us to
avoid the three domains of dysglycemia associated with increased mortality.
Acknowledgements : Jean-Charles Preiser served as consultant or was involved in educational actitivies organised by Edwards, Maquet, Medtronic, Optiscan.






