In vitro study validating the microbial barrier function by means of airborne contamination
Jürgen Gebel, Sapuna Kuriakose, Barbara Grüter, Martin Exner; University of Bonn Hospital, Institute for Hygiene and Public Health, Germany
BACKGROUND
Nosocomial infections pose a major problem in the healthcare system because they contribute to increased morbidity and mortality of hospitalised patients. The most frequently involved bacterial pathogens are Staphylococcus aureus, Pseudomonas aeruginosa, and coagulase-negative staphylococci such as Staphylococcus epidermidis[1]. In addition to bacteria, viruses, parasites, and fungi are also isolated from patients suffering from nosocomial infections.
In the hospital setting, patients can catch infections in three ways:
• From the patient´s own permanent or transient skin micro-flora.
• Exogenous cross-infection due to transfer of microorganisms between patients through direct contact, aerosols, and objects contaminated by the patient's own flora or those of the medical staff[2].
• Endemic or epidemic exogenous infection caused by the flora found in the healthcare setting[2].
This latter type is caused by microorganisms which are well-adapted to the hospital environment[2].
Hospital-acquired infections are often associated with the use of medical devices. Open infusion systems and open drug transfer devices increase the risk of microbial entry leading to infection. To minimise such infections, the National Institute for Occupational Safety and Health (NIOSH) recommends the use of a closed-system transfer device defined as follows[3].
“A drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of hazardous drug or vapour concentrations outside the system”.
PURPOSE
The aim of this study was to evaluate the microbial tightness of Mini-Spike® 2 Chemo V (with integrated Safeflow valve) against airborne contamination.
Mini-Spike® 2 Chemo V is a vented dispensing pin which is used for reconstitutions and drug admixtures.
METHODS
The airborne contamination analysis was carried out using Bacillus subtilis spores. The preparation and purification were performed according to the laboratory standard method described by Gebel in 1998[4]. The Mini-Spike® 2 Chemo V was inserted into a vial containing 50ml of 0.9% sodium chloride solution. The spiked vial was placed together with five 10ml Luer Lock syringes, five Softa® Cloth CHX 2% wipes, and five Combi-Stoppers in an exposure chamber. A nebuliser containing a suspension of Bacillus subtilis spores (average concentration: 5.6 x 103 cfu/m3) was used to generate an aerosol for one minute (Figure 1).
Figure 1: Experimental setup

(A) Safeflow valve integrated in Mini-Spike® 2 Chemo V; (B) Experimental setup;
(C) Withdrawal of NaCl after nebulisation
For an equal distribution of spores after two minutes, the Safeflow valve was disinfected and left to air-dry for 15 seconds. Then a 10ml Luer Lock syringe was filled with 8ml of sodium chloride solution and closed with a Combi-Stopper. Thirty minutes later, the Bacillus subtilis spore suspension was nebulised once again but only for 30 seconds. The entire procedure starting from disinfection of the valve was then repeated with the remaining four syringes. The sodium chloride solution from all syringes and the remaining fluid in the vial were then filtered (through a 0.45µm filter), and afterwards incubated on tryptic soy agar at 37°C for 48 hours.
The entire experimental procedure was repeated a total of three times. Three Mini-Spike® 2 Chemo V devices were tested for each experiment.
RESULTS
No transmission of Bacillus subtilis spores through the valve after contamination of the chamber (with 1.34 x 105 cfu of B. subtilis spores which corresponds to 5.6 x 103 cfu/m3 on average; 95% confidence interval for microbial tightness: 66.4–100%) was detected in any of the Mini-Spike® 2 Chemo V devices tested (Table 1).
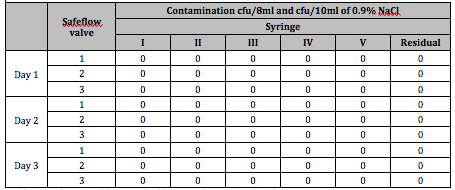
Table 1:Evaluation of the microbial barrier of Mini-Spike®2 Chemo V
CONCLUSION
Currently, very few studies are available concerning the microbial barrier function of closed system transfer devices. Mini-Spike® 2 Chemo V is a closed system device according to the NIOSH recommendations. The acquired data show that the test method used is suitable for investigating the microbial tightness of infusion devices such as Mini-Spike® 2 Chemo V.
In general, the bio-burden in the ambient air of operating theatres and intensive care units ranges from 101 cfu/m3 to 102 cfu/m3 of air[5-7].
This study was carried out using concentrations of Bacillus subtilis spores 100 times higher than those found in ambient air.
The evaluation of the Mini-Spike® 2 Chemo V demonstrated highly effective microbial tightness when applied according to instructions, even when exposed to excessive air contamination.
REFERENCES
[1] Vincent JL et al. The prevalence of nosocomial infection in Intensive Care Units Europe: The results of the EPIC study. JAMA 1995;274:639-44
[2] World Health Organization. Prevention of hospital-acquired infections. WHO/CDS/CSR/EPH/2002.12; 2002
[3] NIOSH Alert: DHHS (NIOSH) Pub No. 2004-165; September 2004
[4] Gebel J. Standardisierung der mikrobiologischen Dosimetrie im Rahmen der Desinfektion von Trinkwasser mit UV-Strahlen. Mathematisch-Naturwissenschaftliche Fakultät. Bonn, Rheinische Friedrich-Wilhelm-Universität: 161;1998
[5] Qudiesat K. Assessment of airborne pathogens in healthcare settings. AJMR 2009;3(2):66-76
[6] Li CS, Hou PA. Bioaerosol characteristics in hospital clean rooms. Sci Total Environ 2003;305(1-3):169-76
[7] Augustowska M, Dutkiewicz J. Variability of airborne microflora in a hospital ward within a period of one year. Ann Agric Environ Med 2006;13(1):99-106
Latest Articles
Staphylococcus aureus, Pseudomonas aeruginosa, Nosocomial infections, microbial tightness, airborne contamination, hospitalised patients, coagulase-negative staphylococci, bacterial pathogens, Staphylococcus epidermidis, Hospital-acquired infections, Mini-Spike®, Mini-Spike® 2 Chemo V, Chemo V, Closed System Transfer Device, afeflow valve
Evaluation of the Microbial Tightness of Mini-Spike® 2 Chemo V - A Closed System Transfer Device






