In vitro study validating the microbial barrier function by means of airborne and touch contamination
Juergen Gebel, Sapuna Kuriakose, Barbara Griiter, Martin Exner; University of Bonn Hospital, Institute for Hygiene and Public Health, Germany
BACKGROUND
Nosocomial infections pose a major problem in the healthcare system because they contribute to increased morbidity and mortality of hospitalised patients.The most frequently involved bacterial pathogens are Staphylococcus aureus, Pseudomonas aeruginosa, and coagulase-negative staphylococci such as Staphylococcus epidermidis[1]. In addition to bacteria, viruses, parasites, and fungi are also isolated from patients suffering from nosocomial infections.
In the hospital setting, patients can catch infections in three ways:
• From the patient's own permanent or transient skin micro-flora.
• Exogenous cross-infection due to transfer of microorganisms between patients through direct contact, aerosols, and objects contaminated by the patient's own flora or those of the medical staff[2].
• Endemic or epidemic exogenous infection caused by the flora found in the healthcare setting[2].
This latter type is caused by microorganisms which are well-adapted to the hospital environment[2].
Hospital-acquired infections are often associated with the use of medical devices. Open infusion systems and open drug transfer devices increase the risk of microbial entry leading to infection. To minimise such infections, the National Institute for Occupational Safety and Health (NIOSH) recommends the use of a closed-system transfer device defined as follows[3].
"A drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of hazardous drug or vapour concentrations outside the system".
PURPOSE
The aim of this study was to evaluate the microbial tightness of the Safeflow Valve against touch and airborne contamination.
Safeflow is a needle-free closed injection port which is used for preparation and administration of intravenous therapies.
METHODS
The touch contamination analysis was carried out using Staphylococcus aureus.
Staphylococcus aureus was prepared and purified according to DIN EN 12353. A lntrafix® SafeSet administration set was inserted into an Ecoflac® plus IV solution container with SOOml of 0.9% sodium chloride solution, and the line was filled. An extension line (“Heidelberger” type) was connected to a single Safeflow valve, and the end of the line was placed into a sterile graduated cylinder.
A suspension of 10(7) cfu/ml of Staphylococcus aureus was used to contaminate the Safeflow valve. The volume of suspension applied per valve was 10ml. This corresponds to 10(5) cfo of Staphylococcus aureus for each valve. After a drying period of one hour, the membrane of the Safeflow valve was then disinfected and let to air-dry. Next, it was connected to the LUer lock of an Intrafix® SafeSet administration set. The roller clamp of the Intrafix® was opened, and 80ml of the sodium chloride solution were transferred from the Ecoflac® plus into the sterile glass. The entire procedure starting with the contamination of the valve was repeated four times. The collected infusatn was filtered and incubated (Figure 1). Nine Safeflow devices were tested.
Figure 1: Experimental setup
(A) Safeflow valve; (B) Experimental setup
RESULTS
No transmission of Staphylococcus aureus through the needle-free access valve after contamination was detected in any of the Safeflow devices tested (Table 1).
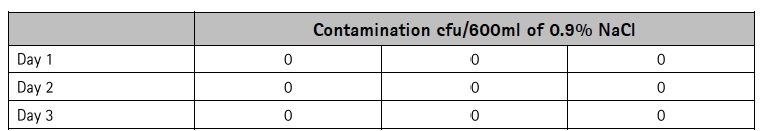
Table 1: Evaluation of the microbial barrier of the Safeflow valve
CONCLUSION
Currently, very few studies are available concerning the microbial barrier function of closed system transfer devices. Safeflow is a closed system according to the NIOSH definition. The acquired data show that the test method used is suitable for investigating the microbial tightness of infusion devices such as the Safeflow valve.
In general, the contamination on the fingertips of physician’s dominant hands reaches on average 18.7 cfu/cm2 of aerobic bacteria[4]. According to Pittet et.al[5] intact areas of some patients’ skin can carry 100-10(6) cfu/cm2, which can serve as a source for microbial transmission onto the healthcare worker’s hands.
This study was carried out with touch contamination of 10(5)-times higher concentration of Staphylococcus aureus. The evaluation of Safeflow demonstrated highly effective microbial tightness based on touch contamination. The excellent results were also found for airborne contamination.
The evaluation of the Safeflow valve demonstrated highly effective microbial tightness when applied/used according to instructions, even when exposed to excessive airborne or touch contamination.
REFERENCES
[1] Vincent JL et.al. The prevalence of nosocomial infection in Intensive Care Units Europe: The results of the EPIC study. JAMA 1995;274:639-44
[2] World Health Organisation. Prevention of hospital-acquired infections. WHO/CDS/CSR/EPH/2002.12; 2002.
[3] NIOSH Alert: DHHS (NIOSH) Pub No. 2004-165; September 2004
[4] Longtin Y et al. Contamination of stethoscopes and physicians’ hands after a physical examination. Mayo Clinic Proc 2014;89(3):291-9
[5] Pittet D et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 2006;6;641-52
Latest Articles
staphylococci, Staphylococcus aureus, Pseudomonas aeruginosa, Nosocomial infections, microbial tightness, airborne contamination, hospitalised patients, Safeflow Valve, touch contamination, bacterial pathogens, Staphylococcus epidermidis, Hospital-acquired infections, Ecoflac®
In vitro study validating the microbial barrier function by means of airborne and touch contamination Juergen Gebel, Sapuna Kuriakose, Barbara Griiter, M...

























