SOLUS is developing a multimodal breast imaging system
involving diffuse optics and ultrasound.
The SOLUS project develops a novel multimodal system that significantly improves the
characterisation of breast lesions.
Breast cancer is one of the most common
cancers in the world. It is estimated that
about one in eight women in Europe will
develop breast cancer before the age of 85 (International
Agency for Research on Cancer 2018; Curado
et al. 2007). The chances for survival increase
substantially upon early diagnosis of breast cancer,
so the availability of diagnostic tools with a high
sensitivity and specificity is vital.
The International Agency for Research on Cancer
has confirmed the effectiveness of mammographic
screening in reducing breast cancer mortality
(Lauby-Secretan et al. 2015). Unfortunately, such
screening programmes return a significant number of
false positive cases (Lancet 2012). These require
further examination, such as additional imaging or
invasive procedures such as biopsies. Approximately
50% of positive breast screening outcomes turn out
to be false positives, meaning that a large number
of additional examinations could have been avoided.
These additional examinations not only have
a negative impact on the patient’s quality of life,
they also represent a high economic burden. Thus,
there is a clear need for an affordable point-ofcare
system with a high specificity to improve the
in-depth characterisation of breast lesions.
This article summarises the rationale behind the
SOLUS project whose aims are the development
of a multimodal breast imaging system involving
diffuse optics and ultrasound.
Ultrasound
Ultrasonography (US) is the first-choice technique to assess the morphology of breast lesions and
guide breast biopsies. Based on the morphological
features of a lesion, a distinction between malignant
and benign lesions is possible using the Breast
Imaging Reporting and Data System (BI-RADS)
(Mendelson et al. 2003). BI-RADS provides standardised
terminology to describe and assess breast
lesions, as well as recommendations for further
follow-up.
BI-RADS category 3 are considered benign
lesions, with a very low rate of malignancy. However,
BI-RADS category 4 covers a wide range of lesions
whose malignancy status is less predictable.
The diagnostic results of conventional US are
frequently unsatisfactory. Improved characterisation
of lesions might allow better BI-RADS categorisation
and decrease invasive follow-ups.
Shear wave elastography
Recently, shear wave elastography (SWE) has
been introduced as an advanced US technique.
SWE provides a quantitative and reproducible measurement of tissue stiffness. Tissue stiffness
can serve as a marker of malignancy, as malignant
tissue generally contains more extracellular matrix,
increasing its rigidity. A recent meta-analysis has
evaluated the performance of SWE for the diagnosis
of breast cancer (Liu et al. 2016). The specificity of
conventional US was 55%. The combination of SWE
and conventional US resulted in a specificity of 80%.
This is a promising increase, but further improvement
in specificity is desirable to achieve a significant
reduction in the false-positive rate.
Diffuse optical imaging
Optical imaging is an appealing candidate as a
method complementary to US. Optical imaging
methods can give insight into tissue composition,
which US is unable to do.
With diffuse optics, a form of optical imaging,
it is possible to measure the light absorption and
scattering properties of tissue. The absorption and
scattering properties of light at different wavelengths
provides information about tissue structure,
composition and functional blood parameters,
such as haemoglobin concentration, oxygen saturation,
and water and lipid content. Diffuse optical
imaging can probe tissue to a depth of a few centimetres,
which makes it suited for the non-invasive
diagnosis of breast cancer.
Cancerous breast tissue is typically characterised
by high haemoglobin and water content, while
lipid content is correspondingly low. High scattering
has also often been detected in malignant lesions
(Durduran et al. 2010; Leff et al. 2008). These observations
all correlate with known changes associated
with tumour development, such as neoangiogenesis,
alterations of stromal components and increased
extracellular matrix deposition.
Collagen can also be measured using diffuse
optics. Alterations in the composition of the extracellular
matrix are well-known aspects of pathological
breast conditions and a causal link between
collagen and tumour formation and progression
has been established (Luparello 2013). Thus, information
on the collagen content in breast tissue
could provide useful information for breast lesion
classification.
Pioneering research on the optical characterisation
of tissue by the Politecnico di Milano, Italy, has
recently shown encouraging preliminary results that
collagen may be even more crucial than haemoglobin
concentration in the differentiation between malignant and benign breast lesions (Quarto et al.
2014; Taroni et al. 2009; Dalla et al. 2015; Konugolu
et al. 2012).
With diffuse optical imaging using multiple
extremely short light pulses at different wavelengths,
a complete optical characterisation of
tissue is possible in a single measurement. However,
its spatial resolution is a well-established limitation.
To better exploit the information from diffuse
optical imaging, and to overcome its limited spatial
resolution, morphologic data obtained from other
imaging modalities, such as mammography, MRI,
PET or US, have been used to provide so-called
prior information for the diffuse optical tomography
reconstruction, or for combined imaging to provide
anatomical landmarks.
Contrary to conventional x-ray mammography or
PET, US does not involve the use of ionising radiation
and does not have many of the disadvantages
of these modalities (complexity, high cost, size of
equipment, long examination times, use of contrast
agents, limited patient acceptance). This makes US
an ideal method from which to derive anatomical
information and to complement diffuse optics.
The SOLUS project
The SOLUS project is developing an innovative,
multimodal tomographic system, combining diffuse
optics, US and SWE, to support the in vivo diagnosis
of breast cancer. Our multimodal system will improve
the classification of breast lesions, more specifically
the discrimination of lesions that are borderline
between benign and malignant (BI-RADS 3 vs.
4a). These presently have high false-positive rates
Combining diffuse optics with US can be achieved
via the development of a portable, cost-effective,
non-invasive, point-of-care diagnostic tool.
The SOLUS project is exploiting innovative
photonics concepts for the development of new
components. By employing diffuse optics with a
small source-detector distance and a time-gated
approach, the SOLUS system will achieve unprecedented
sensitivity, spatial resolution, and depth
penetration, thereby providing effective, diagnostic
information on tissue composition and functional
blood parameters to complement the anatomical
information and characteristics of tissue stiffness
provided by conventional US and SWE, respectively.
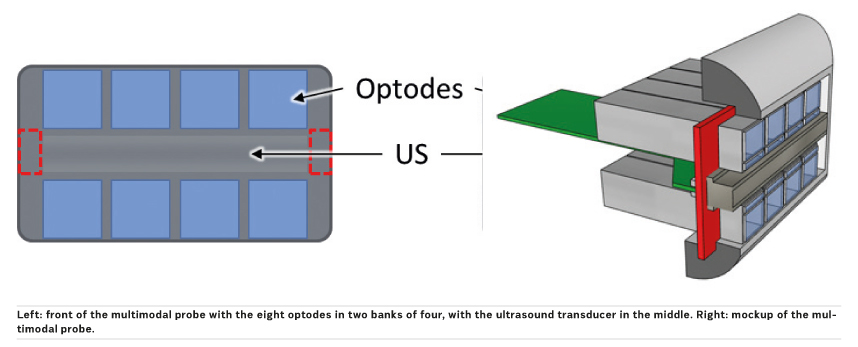
We are developing an innovative photonics
module, called a smart optode to perform the
diffuse optical tomography. The smart optode includes a novel laser driver and newly developed
detector and acquisition electronics. The smart
optode itself will be small in size (measuring about
1 cm2 at the front). Multiple smart optodes will be
combined with a conventional US transducer into
a multimodal probe capable of carrying out diffuse
optical tomography as well as US and SWE measurements
all at once.
This multimodal probe is at the heart of the
SOLUS system for high-specificity, multi-parametric
breast imaging and diagnosis of breast cancer.
The examination procedure will be very similar
to current standard US practices. This facilitates
acceptance by both patients and clinicians.
This multimodal probe is at the heart of the
SOLUS system for high-specificity, multi-parametric
breast imaging and diagnosis of breast cancer.
The examination procedure will be very similar
to current standard US practices. This facilitates
acceptance by both patients and clinicians.
After assessment of the specificity, sensitivity
and spatial resolution of the system in laboratory
trials, we plan to validate the SOLUS system in real
clinical settings. A pilot clinical study on patients
with benign and malignant breast lesions (20 each)
has been designed to demonstrate the overall
feasibility of the proposed approach, the practical
usability of the multi-modal instrument, and at the
same time to provide insights into the real diagnostic
advantages that can be achieved.
Impact of SOLUS
The SOLUS system will achieve substantially
improved breast cancer diagnosis, leading to a
reduction in unnecessary biopsies and decreasing
the economic burden on our healthcare systems. The system will also allow more effective treatment
and therapy management. New and improved
therapy response prediction and monitoring enable
personalised decision-making, therapy planning and
optimisation for each patient. This also contributes
to a significant decrease in the total cost of breast
cancer diagnosis.
Conclusion, first results and
achievements
The project partners are currently finishing the
development of the components for the system.
The overall design of the smart optode has
already been completed. Subcomponents of the
smart optode, such as the compact laser driver and
the time-gated single-photon detector, have been
developed and are currently in the final stages of
testing prior to their integration.
Furthermore, phantoms and protocols for performance
assessment have been completed.
Work on the integration of the multimodal probe
is currently ongoing. The practical ergonomics of
the probe are very important, so special attention
is being paid to feedback from our collaborating clinicians on this aspect.
Highly automated image processing and reconstruction
algorithms are being developed and tested
with promising early results. These use anatomical
information from US for the reconstruction of the
diffuse optics measurements. Additional software
for the operation of the entire system is also under
development.
Ultimately, the multimodal probe will be incorporated
into an existing, commercially available ultrasound
system from project partner SuperSonic
Imagine.
Facts, figures and acknowledgement
The SOLUS project is coordinated by Prof. Paola
Taroni from the Politecnico di Milano, Italy. It started
in November 2016 and will conclude in October
2020. The consortium brings together physicists,
engineers, clinicians and industry partners
to develop the SOLUS system for improved breast
cancer diagnosis. The consortium consists of nine
partners from five European countries:
• Politecnico di Milano, Milan, Italy
• CEA-Leti, Grenoble, France
• SuperSonic Imagine, Aix-en-Provence, France
• Vermon, Tours, France
• University College London, London, UK
• Micro Photon Devices, Bolzano, Italy
• European Institute for Biomedical Imaging
Research, Vienna, Austria
• iC-Haus, Bodenheim, Germany
• Ospedale San Raffaele, Milan, Italy
SOLUS has received funding from the European
Union’s Horizon 2020 research and innovation
programme under grant agreement No 731877.
The SOLUS project is an initiative of the Photonics
Public Private Partnership.
Key points
• A high number of breast lesions, detected by screening programmes, are false-positives.
• Better discrimination between benign and malignant breast lesions is necessary to reduce the number of unnecessary procedures and the economic burden.
• Optical imaging methods provide an excellent addition to conventional ultrasound imaging.
• The SOLUS project is developing an innovative, multimodal tomographic system, combining diffuse optics and ultrasound to support the in vivo diagnosis of breast cancer.
This article was co-authored by:
Alberto Dalla Mora
Associate Professor
Department of Physics,
Politecnico di Milano (POLIMI)
Alberto Tosi
Assistant Professor
Department of Electronics,
Politecnico di Milano (POLIMI)
Milano, Italy
Antonio Pifferi
Professor
Department of Physics,
Politecnico di Milano (POLIMI)
Milano, Italy
Jean-Marc Dinten
Head of CEA-LETI
Grenoble, France
Mathieu Perriollat
Optical systems project manager
CEA-LETI
Grenoble, France
David Savery
Research Engineer
SuperSonic Imagine
Aix-en-Provence, France
Hélène Sportouche
Clinical Product Specialist
SuperSonic Imagine
Aix-en-Provence, France
Bogdan Rosinski
Research Engineer
VERMON
Tours, France
Simon Arridge
Professor
Centre for Medical Image Computing, University College London
London, UK
Andrea Giudice
CTO
Micro Photon Devices
Bolzano, Italy
Simone Tisa
Research Engineer
Micro Photon Devices
Bolzano, Italy
Elena Venturini
Radiologist
San Raffaele Hospital
Milano, Italy
Pietro Panizza
Head of the Breast Imaging Unit
San Raffaele University Hospital
Milano, Italy
Pamela Zolda
European Research Manager
European Institute for Biomedical Imaging Research
Vienna, Austria
Ing. Alexander Flocke
Sales / Application specialist
iC-Haus GmbH
Bodenheim, Germany
Curado M, Edwards B, Shin H, Storm H, Ferlay J, Heanue M, Boyle P (2007) Cancer Incidence in Five Continents. IARC Press, Lyon Vol. 9
Dalla Mora A, Contini D, Arridge S, Martelli F, Tosi A, Boso G, Farina A, Durduran T, Martinenghi E, Torricelli A, Pifferi A (2015) Towards next-generation time domain diffuse optics for extreme depth penetration and sensitivity. Biomedical Optics Express 6:1749.
Durduran T, Choe R, Baker W, Yodh A (2010) Diffuse optics for tissue monitoring and tomography. Reports on Progress in Physics 73: 1
International Agency for Research on Cancer. “Global Cancer Observatory” (2018) Available from gco.iarc.fr.
Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. (2012) The Lancet 380: 1778
Konugolu Venkata Sekar S, Beh JS, Farina A, Dalla Mora A, Pifferi A, Taroni P (2017) Broadband diffuse optical characterization of elastin for biomedical applications. Biophys Chem 229: 130 14.
Lauby-Secretan B, Scoccianti C, Loomis D, Benbrahim-Tallaa L, Bouvard V, Bianchini F, Straif K (2015) Breast Cancer Screening — Viewpoint of the IARC Working Group. New England Journal of Medicine 372: 2353
Leff D, Warren O, Enfield L, Gibson A, Athanasiou T, Patten D, Hebden J, Yang G, Darzi A (2008) A diffuse optical imaging of the healthy and diseases breast: a systematic review. Breast Cancer Research and Treatment 108:9.
Liu B, Zheng Y, Huang G, Lin M, Shan Q, Lu Y, Tian W, Xie X (2016) Breast Lesions: Quantitative Diagnosis Using Ultrasound Shear Wave Elastography—A Systematic Review and Meta-Analysis. Ultrasound in Medicine and Biology 42: 835
Luparello C (2013) Aspects of Collagen Changes in Breast Cancer J of Carcinogenesis & Mutagenesis S13.
Mendelson E, Baum J, Berg W, Merritt C, Rubin E (2003) Breast imaging, Reporting and Data System, BI-RADS: ultrasound. American College of Radiology.
Pearlman PC, Adams A, Elias SG, Mali WP, Viergever MA, Pluim J (2012) Mono- and multimodal registration of optical breast images. J Biomed Opt 17: 080901
Quarto G, Spinelli L, Pifferi A, Torricelli A, Cubeddu R, Abbate F, Balestreri N, Menna S, Cassano E, Taroni P (2014) Estimate of tissue composition in malignant and benign breast lesions by time-domain optical mammography. Biomedical Optics Express 5:3684.
Taroni P, Pifferi A, Salvagnini E, Spinelli L, Teoricelli A, Cubeddu R (2009) Seven-wavelength time-resolved optical mammography extending beyond 1000 nm for breast collagen quantification. Optics Express 17: 15932