By merging stem cell and 'organ-on-a-chip' technologies Harvard scientists have, in a world premiere, successfully grown functioning human heart tissue that carries an inherited cardiovascular disease.
A major development for personalised medicine, this research, which was published in Nature Medicine, provides working proof that laboratory-based replication of a chunk of tissue containing a patient's specific genetic disorder can be achieved.
This project is the collaboration of scientists from the Harvard Stem Cell Institute, Boston Children's Hospital, the Wyss Institute for Biologically Inspired Engineering, Harvard Medical School and the Harvard School of Engineering and Applied Sciences.
Kevin Kit Parker, PhD, provided 'organs-on-chips' expertise, which was combined with the stem cell and clinical insights of William Pu, MD.
The team of investigators used their interdisciplinary approach to model the cardiovascular disease Barth syndrome. This rare X-linked disorder is the result of a single gene mutation called Tafazzin, or TAZ, and currently has no treatment. Linked to a variety of symptoms affecting skeletal and heart muscle, Barth syndrome is primarily prevalent in boys.
The researchers used skin cells from two Barth syndrome patients to manipulate them into becoming stem cells that carried these patients' TAZ mutations. Rather than generating single heart cells in a dish, the team tricked the cells into joining together as they would if they were forming a diseased human heart. This was done by growing them on chips lined with human extracellular matrix proteins that imitates their natural environment. As a result the engineered diseased tissue contracted very weakly, similar to the heart muscle in patients with Barth syndrome.
Using genome editing (a technique that was pioneered by the Harvard collaborator George Church, PhD) in order to achieve TAZ mutation in normal cells, the investigators then confirmed that this mutation was responsible for causing weak contraction in the engineered tissue.
When they delivered the TAZ gene product to diseased tissue a correction of the contractile defect was achieved, this created the first tissue-based model of correction of a genetic heart disease.
With more than ten years of experience in 'organs-on-chips' technology to draw from, Parker pointed out that the meaning of a single cell's genetic mutation was not understood until a large price of organ was reproduced and its functioning, or lack thereof, was evaluated.
He went on to explain that in cells grown out of patients with Barth syndrome, far weaker contractions and irregular tissue assembly was observed. Parker considered the ability to model the disease from one single cell all the way up to heart tissue to be a major advancement.
The scientists were able to discover that although the TAZ mutation was disrupting the normal activity of mitochondria, often referred to as the power plants of the cell for their role in making energy, it was not affecting overall energy supply of the cells. This provided an association between mitochondrial function and a heart cell's ability to build itself in a way that allows it to contract, something previously unknown about mitochondria.
William Pu, who cares for Barth syndrome patients, said that the TAZ mutation made Barth syndrome cells produce an excess amount of reactive oxygen species or ROS, which is a normal byproduct of cellular metabolism released by mitochondria. This development was also unrecognised to date.
Pu added that the team had been successful in showing contractile function could be restored by quenching excessive ROS production in the laboratory setting.
He acknowledged that it was a further challenge to achieve this result in an animal model or a patient, but if feasible, it would provide an innovative therapeutic angle.
The team is now conducting ROS therapy and gene replacement therapy in animal models of Barth syndrome and utilising their human 'heart disease-on-a-chip' as a testing platform for drugs that are potentially under trial or already approved.
According to Parker it had been worthwhile to focus the research on a variety of angles, and he was confident that the technology had provided the university-based researchers and industry with the tools they required to battle the disease.
Parker and Pu both attribute the research success to their scientific consilience and partnership, having first spoken about a collaboration when they attended a conference in Stockholm in 2012.
Parker asserted that the 'organs-on-chips' technology, which has been a flagship of his lab, worked so efficiently because of the high quality of Pu's patient-derived cardiac cells.
Pu concluded that this study was made possible thanks to Harvard being unique in its ability to provide a diverse set of people and cutting-edge technology.
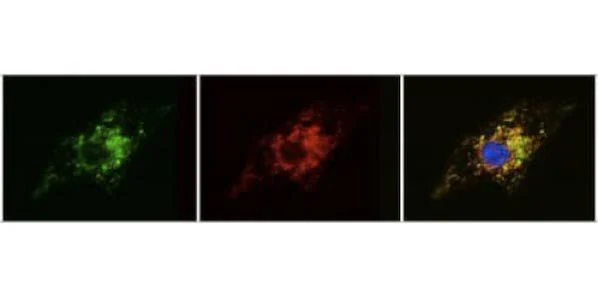
Image caption: Researchers use modified RNA transfection to correct genetic dysfunction in heart stem cells derived from Barth syndrome patients. The series of images show how inserting modified RNA into diseased cells causes the cells to produce functioning versions of the TAZ protein (first image: in green) that correctly localize in the mitochondria (second image: in red). When the images are merged to demonstrate this localization, green overlaps with red, giving the third image a yellow color.
Credit: Gang Wang and William Pu/Boston Children's Hospital
14 May 2014
Latest Articles
Research, laboratory, stem cells, cardio, heart muscle, cardiac cells, gene mutation, Barth syndrome
By merging stem cell and 'organ-on-a-chip' technologies Harvard scientists have, in a world premiere, successfully grown functioning human heart tissue tha...