Fibrostenosis in Crohn’s disease severely affects patients’ quality of life. Current understanding sees intestinal fibrosis as reversible in its early stages but challenging to treat once it progresses. Medical interventions are effective for mild fibrosis, but moderate to severe cases often require endoscopic or surgical approaches. Accurate assessment of fibrosis severity is crucial for selecting appropriate treatments and developing new therapies.
Conventional imaging struggles to reliably detect transmural fibrosis, necessitating cross-sectional methods like magnetisation transfer MRI (MTI), although concerns about consistency remain. Radiomics, extracting detailed features from medical images, offers promise in improving assessment accuracy. A computed tomography enterography (CTE) radiomic model showed efficacy, though MRI is preferred due to radiation risks. Multi-sequence MRI appears superior to CTE for fibrosis evaluation. Texture analysis of contrast-enhanced imaging also shows potential but lacks extensive validation in human studies.
A recent study published in Insights into Imaging aims to develop and validate MRI-based radiomic models for diagnosing intestinal fibrosis in Crohn’s disease, enhancing radiologists' diagnostic accuracy and efficiency.
Development and Validation of MRE-Based Radiomic Models for Intestinal Fibrosis in Crohn’s Disease
From November 2013 to September 2021, a retrospective study included 128 consecutive Crohn’s disease (CD) patients at a single institution. The study, approved by the institutional ethics review board, waived informed consent requirements. Patients met inclusion criteria if they had confirmed CD diagnosis through clinical, imaging, endoscopic, and histological assessments, along with preoperative magnetic resonance enterography (MRE) within three months of surgery for bowel strictures, fistula, or abscess. Exclusions involved poor imaging quality, concurrent bowel diseases, or obscured intestinal contours on MRE.
Patients were randomly divided into training and test cohorts (in a 3:1 ratio) to develop and validate MRE-based radiomic models (RMs). Surgical histopathology evaluated intestinal fibrosis using a region-to-region positioning approach to align MRE findings with histological sections. Histopathology scores categorised fibrosis as none-mild (scores 0-2) or moderate-severe (scores 3-4), ensuring balanced representation in both cohorts for unbiased model development.
MRE protocols employed T2-weighted imaging (T2WI), contrast-enhanced T1-weighted imaging (T1WI), diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC), and magnetisation transfer imaging (MTI). Radiomic analysis involved manually segmenting three-dimensional volumes of interest (VOIs) around bowel lesions, excluding the lumen, and selecting optimal features through recursive feature elimination (RFE). Support vector machines (SVM) were used for binary classification of fibrosis severity (none-mild vs. moderate-severe).
Validation of RMs occurred in an independent test cohort using receiver operating characteristic curves (ROC), Hosmer–Lemeshow goodness-of-fit tests, and integrated discrimination improvement (IDI) and net reclassification improvement (NRI) metrics. Factors affecting diagnostic performance, such as inflammation severity, lesion location, and MRI scanner type, were also assessed.
Visual interpretation criteria were developed based on imaging features from T2WI, T1WI, DWI, ADC, and MTI, with SVM models constructed similarly to the RMs. Inter-observer variability was tested among radiologists for visual interpretation reproducibility. Additionally, a clinical model integrating patient demographics, disease history, and laboratory results was developed using SVM in the training cohort.
The study aimed to enhance diagnostic accuracy for intestinal fibrosis in CD using advanced imaging and clinical data, offering insights into personalised treatment approaches.
Advanced Radiomic Models Enhance Diagnostic Accuracy for Intestinal Fibrosis in Crohn’s Disease
The final cohort included 81 men and 42 women, with an average age of 30.26 years. Patients were divided into training (n = 93) and test (n = 30) cohorts. Bowel segments with fibrotic strictures of varying severity were assessed.
Radiomic feature selection via recursive feature elimination identified 50 fibrosis-related features from each MRE sequence, demonstrating high inter- and intra-observer reproducibility (ICC 0.969–0.998 and 0.977–0.985, respectively). The top five features and their contributions from each MRE sequence were identified.
The diagnostic performance of three radiomic models (RM1, RM2, and RM3) was evaluated in both training and test cohorts using AUC analysis. The accuracy increased from RM1 (AUC 0.86-0.89) to RM3 (AUC 0.91-0.95). RM3 exhibited the highest efficacy, confirmed by Hosmer-Lemeshow tests indicating robustness in both cohorts.
Subgroup analyses revealed consistent RM performance across different bowel segments and MRI scanners. Visual models 1 and 2 had poor performance, while visual model 3, incorporating MTI, showed improved diagnostic accuracy (AUC 0.77 in both cohorts).
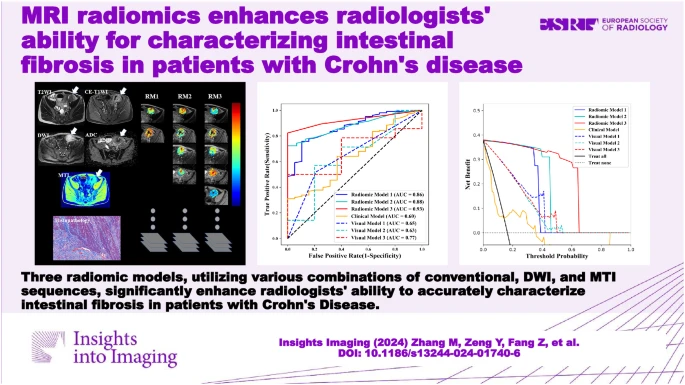
Image Credit: Insights into Imaging
Inter-observer agreement for MRE features varied, with some showing poor agreement (ICC < 0.5) and others good agreement (ICC > 0.75). A clinical model based on CRP and ESR failed to distinguish fibrosis degrees effectively.
Comparison with visual interpretation and clinical models showed superior performance of RMs in both training and test cohorts (all p < 0.05), as confirmed by decision curve analysis demonstrating better predictive benefits for intestinal fibrosis.
Overall, the study underscores the utility of MRE-based radiomics for accurate and reliable assessment of intestinal fibrosis in Crohn’s disease, outperforming visual interpretation and clinical models.
Successful MRE Radiomics for Distinguishing Intestinal Fibrosis
Three multi-sequence MRE-based radiomic models (RMs) were developed and evaluated for their efficacy in distinguishing moderate-severe from none-mild intestinal fibrosis. RM1, utilising basic MRI sequences, demonstrated moderate diagnostic accuracy, which improved with the addition of more specific sequences (DWI or MTI) in RM2 and RM3, showing excellent diagnostic performance.
These RMs were validated in both training and test cohorts, as well as across various subgroups, indicating high reliability and stability. They outperformed visual models based on traditional imaging interpretation and a clinical model using CRP and ESR levels. The inclusion of MTI in RM3 particularly enhanced diagnostic efficacy by quantifying macromolecule concentrations reflective of collagen deposition in bowel walls.
Radiomic analysis of MRE images provided objective and reproducible measurements of intestinal fibrosis, overcoming limitations of conventional imaging, which often fails to assess fibrosis accurately or yields inconsistent results. Interobserver consistency was good for radiomic features, ensuring reliable classification across different inflammatory conditions and anatomical locations.
The study highlighted the potential of RMs to significantly improve radiologists' ability to assess fibrosis severity in CD patients. Despite limitations such as selection bias and the need for standardised MRE protocols, the findings underscored the feasibility and necessity of future multicenter studies to validate these promising results. Multi-sequence MRE-based RMs offer a robust approach for characterising intestinal fibrosis in CD, accommodating varying technical sourcapabilities, and enhancing diagnostic precision in clinical settings.
Source: Insights into Imaging
Image Credit: iStock