About us
Abionic, an emerging medical diagnostics company focused on rapid early detection technologies, received 510(k) clearance for its PSP test from the U.S. Food and Drug Administration (FDA) to accelerate the Time-to-Detection of Sepsis in critical care and emergency settings. Already certified under the EU IVDR as of July 2022, this FDA clearance marks a pivotal moment for Abionic’s expansion into the U.S. market.
Vision
Abion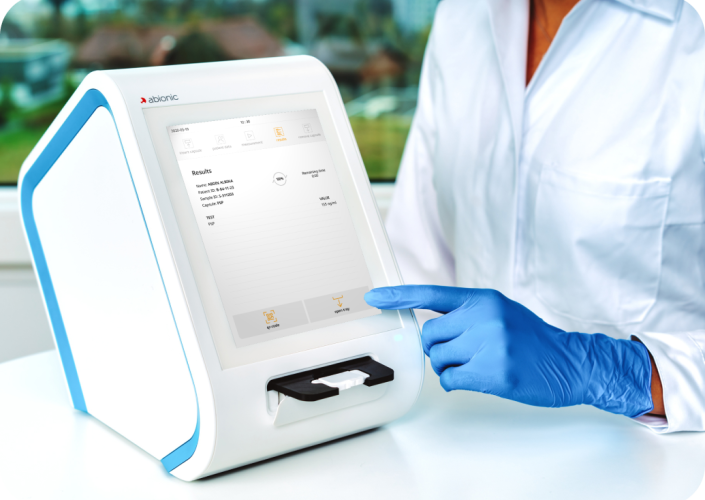 ic’s “First-in-Class” Rapid Testing solutions will become the Gold Standard for near-patient Early Sepsis Detection across Hospital workflows.
ic’s “First-in-Class” Rapid Testing solutions will become the Gold Standard for near-patient Early Sepsis Detection across Hospital workflows.
Iwan Märki, CTO and co-founder of Abionic
Certificates
At Abionic, regulatory compliance is at the heart of everything we do. Our certifications, including FDA 510(k) clearance, ISO 13485, CE Mark, and IVDR compliance, demonstrate our dedication to meeting the highest standards of quality, safety, and performance.
- ISO Certificate
- CE Certificate
- IVDR QMS
Team
Meet the people behind the innovation. Our team combines deep scientific knowledge, technical expertise, and a shared passion for advancing diagnostics. From research and development to clinical collaboration, each member contributes to creating solutions that improve patient care and support healthcare professionals worldwide.
Management
 |  |  |
Patrick Pestalozzi Chief Executive Officer | Iwan Märki Chief Technical Officer & Co-Founder | Guillaume Haquette Chief Biology Officer |