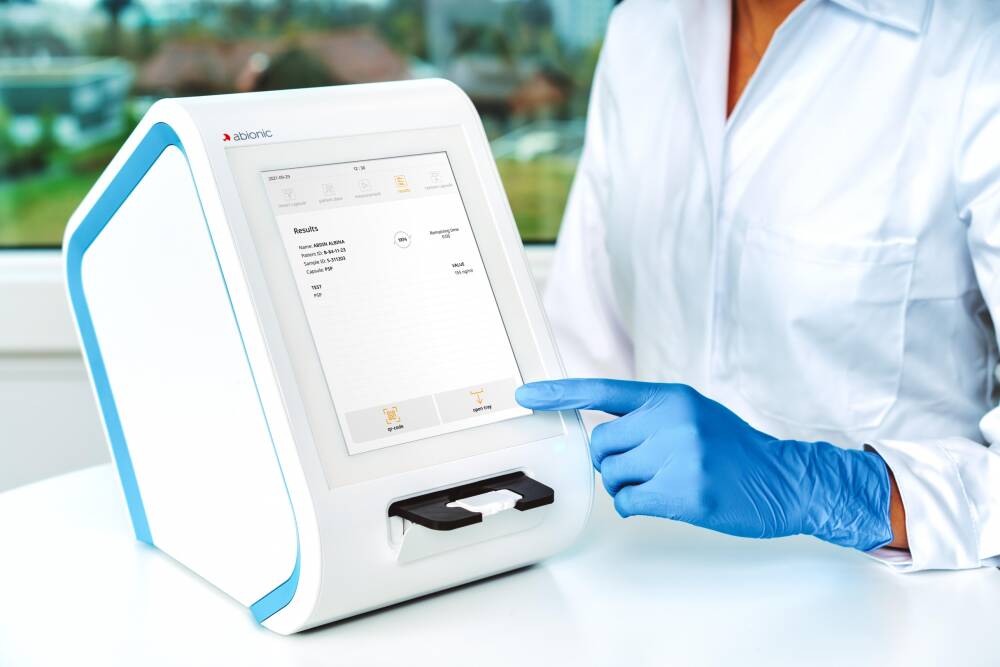
Abionic’s nanotechnology is transforming today’s blood-testing standards by bringing laboratory quality to near-patient testing and simultaneously analysing up to 14 parameters from a single drop of blood, to provide actionable results in just 5 minutes.
By driving molecules into a nanochannel and limiting the travel distance to a few hundred nanometres, molecular interactions are accelerated rapidly. Thus, biomarker levels of e.g. antibodies and proteins can be efficiently quantified within an extremely short assay time, with high precision and accuracy on a closed, small and easy-to-operate platform.
Unparalleled technology offers patients the fastest point-of-care diagnostic testing solutions based on traditional immunoassays
abioSCOPE provides highly accurate, actionable results at the point of care
abioSCOPE requires minimal training and its step-by-step on-screen process is designed for anyone to use
All our tests are run with just a few μl of blood
abioSCOPE does not require regular maintenance. Its corresponding IVD CAPSULES are factory-calibrated.
Results are immediately and automatically transferred to the hospital information system and integrated into patient medical file.
Single-use capsules eliminate the risk of contamination and ensure maximum quality between runs
Fast. Easy. Reliable.
ACUTE CARE SETTINGS:
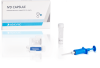
IVD CAPSULE PSP
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the quantitative measurement of pancreatic stone protein (PSP) in blood. The test is intended to be used in conjunction with other clinical assessments and laboratory findings to aid in the early detection of nosocomial sepsis in adults.
Download PSP Product leaflet: https://www.abionic.com/sites/default/files/pdf/D4haab_210610_PSP_Brochure_JGA.pdf
IVD CAPSULE cSOFA
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the determination of Pancreatic Stone Protein (PSP) in blood for the calculation of the cSOFA score at the point of care. The cSOFA score is used to assess the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in adults.
Download cSOFA Product leaflet: https://www.abionic.com/sites/default/files/pdf/D4haadbb_201124_COVID_19_EN_V1.0_JGA.pdf
Loading...