ICU Management & Practice, Volume 23 - Issue 4, 2023
Xenios sponsored a lunch symposium at the 16th European Conference on Paediatric and Neonatal Ventilation (EPNV) in Montreux (CH) in May 2023. Matteo Di Nardo (Italy) introduced the audience to extracorporeal membrane oxygenation (ECMO) in neonates. Florian Kipfmüller (Germany) presented current knowledge and his experience with ECMO in neonates with congenital diaphragmatic hernia (CDH). Heleen van Ommen (Netherlands) shared expert knowledge on coagulation management in neonatal ECMO. This article provides an overview of their talks and discusses the use of ECMO in neonates with CDH and persistent pulmonary hypertension (PPHN) in neonates.
ECMO in Neonates: An Overview
Extracorporeal membrane oxygenation (ECMO) is a form of life support used in neonates and children with life-threatening heart and/or lung problems. The use of neonatal ECMO is indicated in newborns with severe and reversible respiratory and/or cardiac failure with an estimated mortality risk of >80% and who are unresponsive to conventional therapies like surfactant, high-frequency oscillatory ventilation (HFOV), inhaled nitric oxide (iNO), medications for pulmonary hypertension, and medications for acute cardiac failure (Stolar et al. 1991).
The most frequent pathologies treated with ECMO in neonates include Meconium Aspiration Syndrome (MAS), Congenital Diaphragmatic Hernia (CDH), Persistent Pulmonary Hypertension in Neonates (PPHN) and sepsis and septic shock. In recent years, there has been an increase in ECMO runs for cardiac disorders. The most frequent pathologies that are reported for cardiac ECMO include Hypoplastic Left Heart Syndrome (HLHS), cyanotic heart disease with decreased pulmonary flow, cyanotic heart disease with increased pulmonary flow, cardiomyopathy and myocarditis (ECLS Registry Report: International Summary 2022).
ECMO Cannulation
The choice between VA (venoarterial) and VV (venovenous) support for neonatal respiratory ECMO relies on various factors, including the patient's condition, the surgeon's expertise, and the capabilities of the medical centre. When considering cannulation methods, both open cut-down exposure for VA and VV cannulation and percutaneous VV cannulation are valid options, provided they are carried out safely by knowledgeable professionals (Wild et al. 2020). Neck cannulation in neonates is the most common access and used with VA ECMO . There are other types of cannulation, but the use of a double-lumen cannula through the neck is common for VV ECMO .
The ECMO circuit must be downsized to fit the neonatal population. Neonates have immature haemostat systems with an increased risk of bleeding and clotting. They also have fragile red blood cells with immature pfHb learning mechanism. ECMO cannulas in children take up a larger portion of the vessel cross-sectional area than in adults. This becomes especially challenging in infants as their femoral vessels are relatively underdeveloped, making cannulation technically difficult or even impossible. Furthermore, younger children have less developed coagulation systems, including lower antithrombin levels. They are also more susceptible to intracranial haemorrhage due to their delicate germinal matrices. As a result, they are at a higher risk of experiencing complications related to inaccurate anticoagulation and mismanagement of blood products (Butt and MacLaren 2016).
Factors that could potentially influence the outcome in case of oxygenator failure include the severity of the illness, the size of the clot relative to the size of the oxygenator, the availability of a primed circuit and the speed and ease with which a new oxygenator can be primed. For neonates, it is important to improve the design of the oxygenators and ECMO circuits and to allow for adjustment of coagulation parameters to reduce the risk of oxygenator failure (Khoshbin et al. 2005).
There is controversy regarding the ideal pump type for neonatal ECMO, and studies show variation in outcomes with centrifugal pumps and roller pumps. A study comparing conventional roller or centrifugal pumps during neonatal VV ECMO found greater odds of survival with conventional roller pumps (Ündar et al. 2023). This could be due to the different circuit designs. The roller pump is occlusive and overcomes downstream resistance, thus maintaining the same flow rate regardless of changes in pressure. The centrifugal pump, on the other hand, is nonocclusive, and the flow rate depends on rotation speed and downstream resistance. This could lead to haemodynamic variation (Ündar et al. 2023). Roller pumps are associated with fewer episodes of haemolysis and mechanical, cardiac and renal complications. Hence, ECMO with roller pumps may be associated with lower mortality in children and fewer complications.
ECMO in Neonates With PPHN/CDH
Outcome data from the ELSO Registry shows that neonatal respiratory ECMO has a 73% survival to discharge rate (ECLS Registry Report: International Summary 2022). CDH is a major indication, with a high mortality of 41%. In CDH, there is a risk of PPHN and changes in the airway and cardiac function (ESLO Registry Data). The survival rate of paediatric patients with CDH treated with ECMO has changed minimally over the last few decades. The recent CDH population has demonstrated a higher risk profile than before because prenatal diagnosis enables timely treatment of severe cases who would die otherwise. Overall, clinical evidence suggests that ECMO can help infants with CDH who may not survive otherwise (Yu et al. 2019).
ECMO serves as an emergency treatment that sustains the functioning of the heart and lungs, facilitating recovery from reversible respiratory issues. Neonates with CDH often experience varying levels of insufficient lung function. The UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation (UK Collaborative ECMO Trial Group 1996) demonstrated the advantages of ECMO in neonates compared to continued intensive conventional management, such as improved survival rates and reduced occurrences of neurodevelopmental disabilities at one year of age. ECMO has consistently improved the survival of neonates with respiratory failure, with many growing up to enjoy a comparable quality of life to continued intensive conventional management. Infants diagnosed with CDH frequently due to pulmonary hypertensive crisis are considered suitable candidates for ECMO (Yu et al. 2020).
Cardiac dysfunction is a key component of the clinical presentation of severe CDH. Biventricular dysfunction can reduce cardiac output and shunting, leading to systemic hypotension, acidosis and hypoxaemia. This can further exacerbate cardiac dysfunction and pulmonary vasoconstriction. Therefore, it is important to consider the contribution of this dysfunction and elevated PVR and pulmonary hypoplasia. Early assessment of cardiac function and pulmonary artery pressure is essential to prevent clinical deterioration (Patel et al. 2020). A study by Patel et al. (2019) showed that ventricular dysfunction in CDH patients was associated with increased mortality. Left ventricular dysfunction was associated with more severe consequences than right ventricular dysfunction. Biventricular dysfunction had a 49% mortality, while those with a normal or only right ventricular dysfunction had a 27% mortality. Therefore, early ventricular function should be routinely evaluated in cases of CDH to improve outcomes (Patel et al. 2019).
VA ECMO vs VV ECMO – Which is Superior in CDH patients?
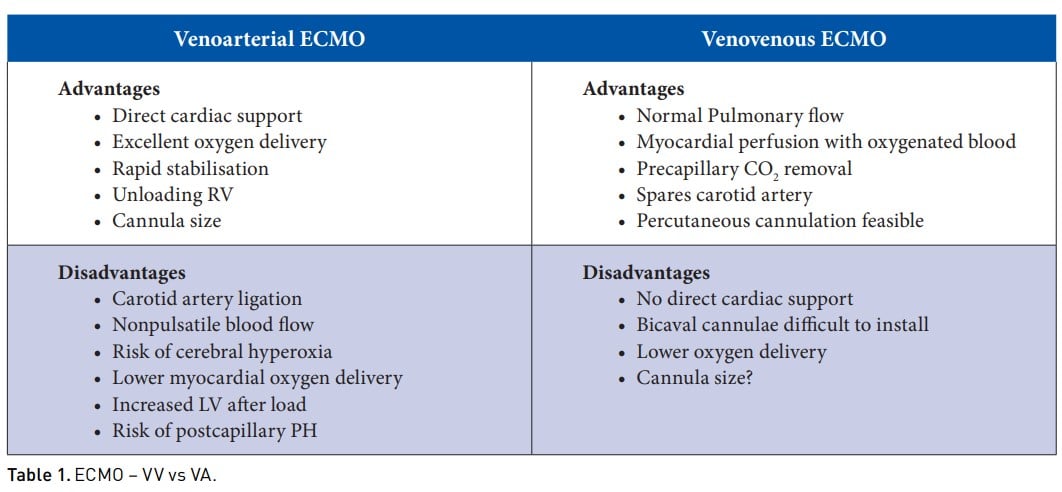
As with all procedures, it is important to consider the advantages and disadvantages of different ECMO cannulation modalities. VA ECMO provides direct cardiac support, excellent oxygen delivery and rapid stabilisation. At the same time, there is a risk of carotid artery ligation, cerebral hyperoxia, lower myocardial oxygen delivery, increased LV afterload and risk of post-capillary pulmonary hypertension. In contrast, VV ECMO helps maintain a normal pulmonary blood flow, ensures myocardial perfusion with oxygenated blood, precapillary CO2 removal, spares the carotid artery and makes percutaneous cannulation feasible. The disadvantages of this cannulation include no direct cardiac support, lower oxygen delivery, and the bicaval cannula can be difficult to install.
VA ECMO is used more commonly in neonates with CDH than VV ECMO . In a study conducted in 2009 with 4115 neonates requiring ECMO, there was no difference in mortality between VV vs VA (Guner et al. 2009). Renal complications and on-ECMO inotrope use were more common with VV, while neurologic complications were more common with VA. Overall, the short-term outcomes of VV and VA were comparable (Guner et al. 2009). The same study was repeated in 2018 and revalidated that the mode of ECMO does not significantly affect mortality or severe neurologic injury in infants with CDH (Guner et al. 2018).
In conclusion, there is no data to support one mode over the other. Poor neurological outcome is associated with VA ECMO; cardiac complications are mainly similar between the two approaches. There may also be issues related to experience and availability of equipment. VV cannulation may also be more time-consuming, and a phenotype-based approach may be the future, but more data is needed before giving any recommendations.
Key Points
- Extracorporeal membrane oxygenation (ECMO) is a form of life support used in neonates and children with lifethreatening heart and/or lung problems.
- VA ECMO provides direct cardiac support, excellent oxygen delivery and rapid stabilisation.
- VV ECMO helps maintain normal pulmonary blood flow, ensures myocardial perfusion with oxygenated blood, precapillary CO2 removal, spares the carotid artery and makes percutaneous cannulation feasible.
- The choice between VA and VV ECMO relies on various factors, including the patient's condition, the surgeon's expertise, and the capabilities of the medical centre.
- The ECMO circuit must be downsized to fit the neonatal population as they are at a higher risk of experiencing complications.
Disclaimer
Point-of-View articles are the sole opinion of the author(s) and they are part of the ICU Management & Practice Corporate Engagement or Educational Community Programme.






