In recent breakthrough research presented during a Hot Line session at ESC Congress 2023, semaglutide's effectiveness in improving symptoms and physical function related to heart failure and inducing greater weight loss was reported.
Nearly 50% of heart failure patients are affected by heart failure with preserved ejection fraction (HFpEF). Most of these patients are overweight or obese. Obesity and excess body fat might play a crucial role in the progression and development of HFpEF rather than just being comorbid conditions. Patients with obesity related HFpEF bear a particularly heavy burden of incapacitating symptoms (like shortness of breath, exertional intolerance, swelling/oedema) and physical limitations, significantly impacting their quality of life. Unfortunately, the available treatment options are limited, and no approved therapies specifically target the obesity-related aspect of HFpEF.
Semaglutide, a potent glucagon-like peptide-1 receptor agonist, has previously demonstrated substantial weight loss benefits for individuals who are overweight. The STEP-HFpEF trial was designed to test whether semaglutide could significantly improve symptoms, physical limitations, exercise function, and weight loss for patients with HFpEF and obesity.
The study was conducted at 96 sites across 13 countries in Asia, Europe, North America, and South America and included 529 patients with a median age of 69 years. Study participants had HFpEF with a left ventricular ejection fraction ≥45%, a body mass index (BMI) ≥30 kg/m² with heart failure symptoms and functional constraints (New York Heart Association functional class II–IV and Kansas City Cardiomyopathy Questionnaire Clinical Summary Score [KCCQ-CSS] <90 points).
Participants received either subcutaneous semaglutide 2.4 mg once weekly or a placebo over a period of 52 weeks. The primary endpoints included the change from baseline to week 52 in KCCQ-CSS and body weight. Secondary outcomes included changes in the 6-minute walk distance (6MWD), heart failure events, changes in KCCQ-CSS and 6MWD, and change in C-reactive protein.
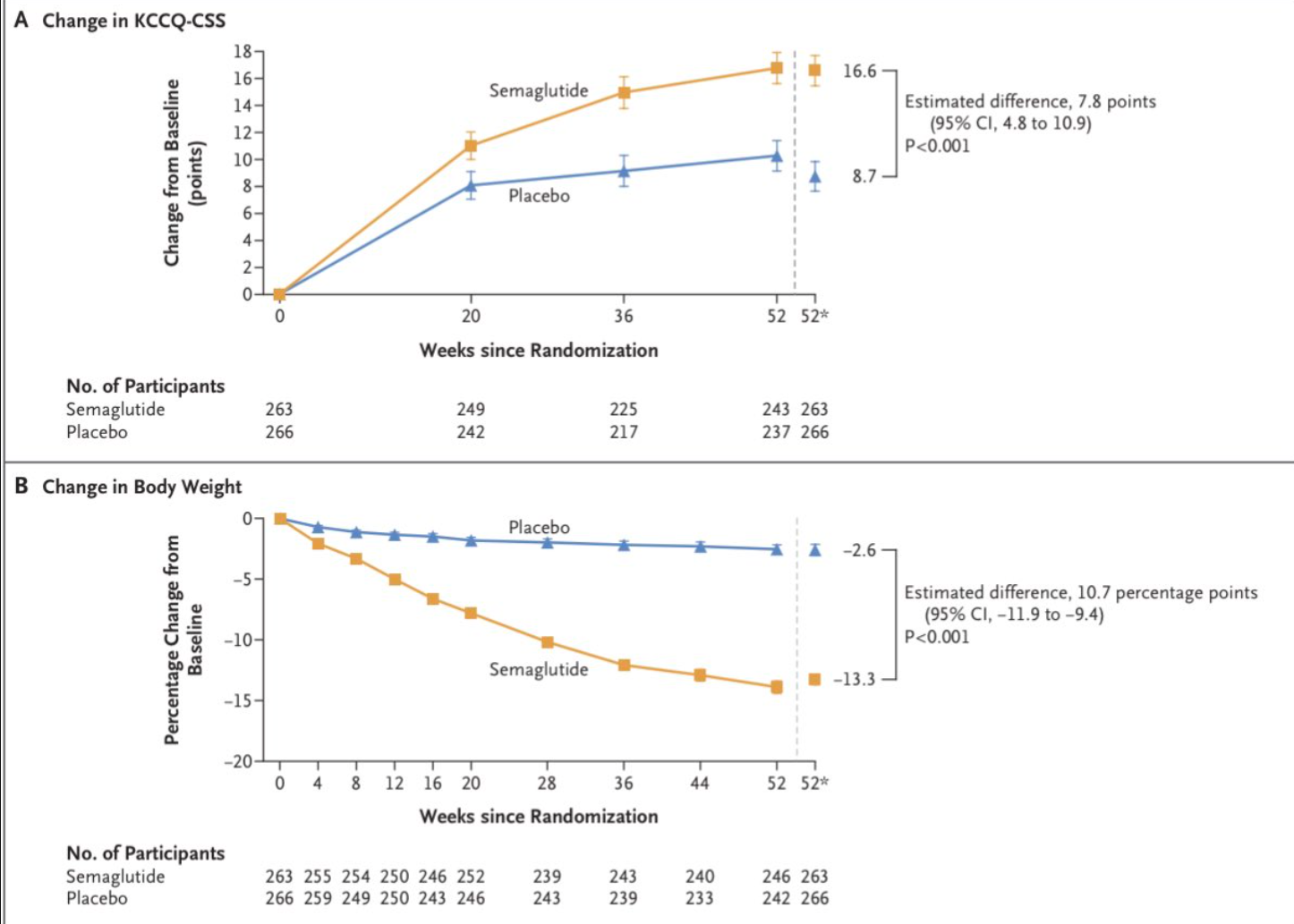
As per the findings of the study, semaglutide led to a mean KCCQ-CSS improvement of 16.6 points from baseline to week 52, compared to 8.7 points with placebo. The mean weight reduction was -13.3% with semaglutide versus -2.6% with placebo. Notable improvements were also observed in 6MWD, CRP levels, and other exploratory endpoints. Serious adverse events were reported in 13.3% and 26.7% of participants with semaglutide and placebo, respectively.
Dr Mikhail Kosiborod, the principal investigator from Saint Luke's Mid America Heart Institute in Kansas City, U.S., highlighted that the trial's results are transformative. He emphasised that semaglutide's efficacy in targeting obesity related HFpEF is groundbreaking, ushering in a new era in managing this patient group where effective therapies are currently lacking. This research underscores that obesity is more than a comorbidity—it is an integral factor contributing to HFpEF and a valid target for therapeutic intervention.
Source: ESC Congress 2023
Image and Slide Credit: ESC Congress 2023



























