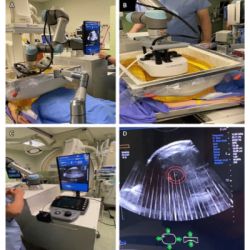
Kodak has applied to the U.S. Food and Drug Administration (FDA) for approval of its CR system for mammography and the company is currently conducting clinical trials at several sites in the U.S. and Canada.
The mammography feature equips healthcare facilities to achieve cost and workflow efficiencies by capturing mammography and general radiography exams with the same computed radiography (CR) platform. Capturing mammography images does require the use of specialized KODAK mammography cassettes and screens.
The following facilities are among those that recently purchased Kodak