Tissue ablation techniques are increasingly important in medical interventions, offering minimally invasive options for treating focal malignancies. These procedures, guided by precise imaging, reduce morbidity and mortality compared to surgical resections. They include energy-based methods like high-intensity focused ultrasound (HIFU) and non–energy-based methods such as histotripsy, which destroy tissue through mechanical effects. Histotripsy, developed at the University of Michigan, uses short ultrasound bursts to create mechanical cavitation, turning targeted tissue into acellular debris. Unlike thermal methods, it minimises heat-related limitations and offers potential across various tumour types, showing promise in preclinical studies. A review published in Radiology: Imaging Cancer offers insight into what histotripsy can bring to clinical practice.
Understanding the Mechanisms and Applications of Histotripsy in Tissue Ablation
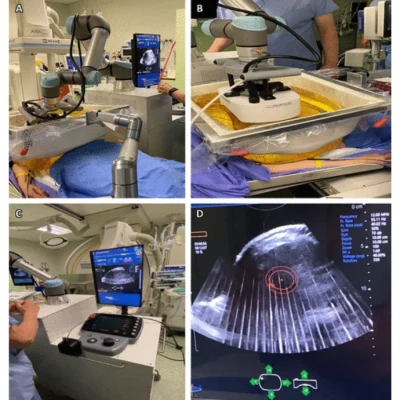
Histotripsy utilises acoustic waves interacting with nanometer-scale gas pockets in tissues to induce cavitation, crucial for tissue ablation. Control of acoustic waves, delivered as microsecond-length pulses at specific negative pressures, is essential for efficacy. Cavitation threshold varies by tissue type, influenced by factors like gas pocket size and distribution. Histotripsy employs three mechanisms to create cavitation bubbles: intrinsic threshold, shock scattering, and boiling histotripsy. Multiple pulses are needed for complete tissue breakdown. Frequencies range from 250 kHz to 3 MHz, with low-duty cycles minimising heat generation. Precise control of intensity parameters ensures effective tissue ablation with minimal thermal effects, making histotripsy promising for various medical applications. In solid tissues, histotripsy transforms tissue into liquid homogenate, while at tissue-fluid interfaces, it erodes the tissue surface, causing sloughing of cell debris. Tissues with high tensile strength, like bile ducts and arteries, resist histotripsy, suggesting potential vessel-sparing ablation strategies. Oedema and fluid uptake observed during histotripsy may have implications for therapeutic drug delivery. In vivo investigations in pig livers showed no histologic evidence of vessel damage, with larger vessels remaining unharmed. Particle size analysis of tissue debris indicates a minimal risk of embolisation. The histotripsy device used in liver tumour clinical trials is developed by HistoSonics and involves a treatment cart and ultrasound machine, with procedures conducted under general anaesthesia in an angiography suite. Histotripsy analysis utilizes various imaging modalities, with ultrasound (US) imaging being beneficial immediately post-procedure, showing hypoechoic areas contrasting with the hyperechoic features during treatment. Some tumours may be better identified via MRI or CT, necessitating fusion with US for accurate targeting. MRI effectively evaluates posttreatment effects, with histotripsy appearing hypointense on T1-weighted and contrast-enhanced MRI. Apparent diffusion coefficient mapping shows histotripsy as a hypointense area, particularly in in vivo studies.
Efficacy and Safety of Histotripsy for Liver Tissue Ablation
Preclinical experimental trials demonstrate the efficacy and safety of histotripsy for liver tissue ablation. In vivo studies in porcine and rodent liver tumours show complete fractionation of hepatic parenchyma with no substantial damage to blood vessels, gallbladder tissue, or overlying structures. Histotripsy's mechanical cavitation mechanism reduces thermal damage to overlying tissues, with newer pulse sequence software showing efficacy in mitigating thermal injury. Safety evaluations indicate no need for systemic heparinization before histotripsy procedures, with no substantial safety concerns regarding large hepatic veins. Long-term studies in rodent models show effective reduction of local tumour burden with no recurrence, possibly due to immunostimulatory effects. Further research is warranted to understand histotripsy's immunomodulatory mechanisms fully.
Challenges in Histotripsy Treatment of Pancreatic Adenocarcinoma
Histotripsy shows promise in treating pancreatic adenocarcinoma, a disease with a dismal prognosis and often diagnosed at advanced stages. In murine models, histotripsy alters the tumour microenvironment, increasing serum concentrations of neoantigens and immune activation, leading to improved survival rates compared to thermal ablation methods. However, challenges arise due to the pancreatic anatomy, limiting the effectiveness and precision of histotripsy. Orthotopic swine studies reveal difficulties in ablating deeper tumours, but a gas-reducing diet improves visualisation and precision. Accurate acoustic property values for pancreatic tissues are crucial for treatment planning, and recent studies have addressed this gap, providing comprehensive data for better execution of US-mediated procedures, particularly thermal therapies.
Exploring Histotripsy for Renal Tumour Treatment
Histotripsy's efficacy in renal tissue has been extensively investigated in rabbit and porcine models, aiming to spare the urinary collecting system while achieving precise tissue destruction. In rabbit kidney studies, histotripsy produced elliptical cavities with smooth boundaries and liquefied centres, sparing perinephric tissue and overlying structures. When targeting different renal areas, histotripsy effectively spared the collecting system while causing cortical tissue liquefaction and moderate damage to the medulla. Studies on VX-2 tumours implanted in rabbit kidneys showed promising results, with histotripsy achieving complete fractionation of targeted tumour zones in 80% of cases. However, histotripsy did not significantly increase the incidence of metastatic lung foci compared to controls, indicating its safety in this context.
Safety and Feasibility Studies around Histotripsy for Prostatic Tissue Ablation
Histotripsy showed promise in treating prostatic tissue in canines, reducing prostatic volume while sparing the urethra and prostatic capsule. Studies have assessed the damage thresholds of surrounding tissues, highlighting the high threshold of the urinary sphincter and neurovascular bundles compared to rectal tissue. Investigations into histotripsy's outcome in anticoagulated models reveal mild haematuria but no significant changes in haemoglobin concentration, although complications such as rectal erosions and prostatic capsule perforations were observed. Boiling histotripsy (BH), a focused ultrasound technology, demonstrates feasibility in ablation of fresh human prostate tissue, suggesting its potential as a clinical option for prostate ablation. BH also shows promise in nonthermal mechanical ablation of human prostate adenocarcinoma tissue, with future studies focusing on protocol optimization for complete tissue destruction.
Efficacy and Immunomodulatory Effects of Histotripsy for Breast Tumour Treatment
Histotripsy has shown effectiveness in destroying breast tumours, with experiments in mouse models revealing the eradication of targeted areas and the development of an anti-tumour microenvironment (TME). In vitro studies using boiling histotripsy (BH) in human breast adenocarcinoma cells demonstrate increased release of damage-associated molecular patterns and proinflammatory cytokines. A novel approach combining dual-frequency mode with two-stage millisecond-length ultrasound pulses has been introduced for treating large-volume triple-negative breast cancer (TNBC) in mice. This method results in controllable-sized lesions, reduced scanning points, stagnant tumour growth, extended survival, and increased immune responses. Studies suggest the potential of this approach as a non-invasive and efficient TNBC treatment, enhancing immune responses.
Exploring Histotripsy as a Safe and Effective Treatment for Brain Tumours
Research has investigated histotripsy as a potential non-invasive treatment for brain tumours. Studies demonstrated histotripsy's capability to ablate deep brain structures and shallow locations with minimal temperature increase. In vivo experiments in healthy porcine brain showed the successful creation of ablation zones without haemorrhage or oedema, indicating initial safety. Recent studies explored different histotripsy dosages in murine brain tumour models, finding that fewer pulses at fewer focal locations effectively eliminated tumours while reducing the risk of haemorrhage.
Histotripsy as a Promising Non-Invasive Approach for Treating Musculoskeletal Tumours
Histotripsy has shown promise as a non-invasive treatment option for musculoskeletal tumours, including osteosarcoma. In ex vivo studies with canine osteosarcoma tumours, histotripsy effectively ablated both soft tissue components and neoplastic cells without harming adjacent nerve tissue or healthy bone samples. Similar results were observed in preclinical murine models, where histotripsy treatment led to the loss of cellular architecture in targeted tumour regions and increased presence of immune cells, suggesting potential improvements in clinical outcomes. Ongoing research is exploring histotripsy's feasibility for treating spontaneous soft tissue sarcoma in vivo, with promising results indicating its potential as a precise and non-invasive treatment option. Recent in vivo studies in cats have further demonstrated the safety and feasibility of using histotripsy to target and ablate superficial soft tissue sarcomas, paving the way for clinical development of histotripsy devices for this application.
Exploring the Promising Future of Histotripsy in Oncology
The future of histotripsy in oncology holds several promising directions. Firstly, understanding the mechanisms of immunomodulation is crucial. Histotripsy has the potential to not only reduce tumour burden but also induce an antitumour immune response by releasing tumour antigens, leading to systemic tumour destruction. Manipulating the tumour microenvironment (TME) to promote proinflammatory states and reduce immunosuppression can further enhance histotripsy's effectiveness. The abscopal effect, where local therapy induces systemic antitumor responses, is another area of interest. Histotripsy has demonstrated this effect in preclinical models, leading to reduced tumour growth and metastasis at untreated sites. This effect may be potentiated when combined with immunotherapy checkpoint inhibitors, suggesting a synergistic approach for enhancing treatment outcomes. Robot-assisted histotripsy represents a promising advancement. By integrating robotics, targeting errors due to respiratory motion can be minimised, enhancing treatment precision and efficiency. This approach holds significant potential for improving therapeutic outcomes, especially in areas influenced by motion, such as the liver and kidneys. Overall, future research endeavours in histotripsy aim to optimise treatment strategies, understand immunomodulatory mechanisms, and explore synergistic approaches with immunotherapy. These efforts have the potential to revolutionise oncologic treatment by providing effective, non-invasive, and immunomodulatory therapies for various types of tumours.
Source & Image Credit: Radiology: Imaging Cancer