HealthManagement, Volume 12 - Issue 2-3, 2012
Imaging modalities nowadays produce a wide range of information contained in high-quality digital medical images. This massive amount of data and technical developments in informatics have facilitated the use of computational image processing methods with the aim to aid in diagnosis and therapeutic decision-making. The combination of high-quality images with such computing techniques allow for the in vivo extraction of different parameters that are sensitised to different biological, physiological, chemical or physical conditions. These parameters may become innovative imaging biomarkers if their variations under healthy conditions are properly analysed and controlled, and if their values are proven to be sensitive and accurate to evaluate the pathologic process, assessing the main clinical endpoints in a surrogate way.
Which Biomarkers are Clinically Useful?
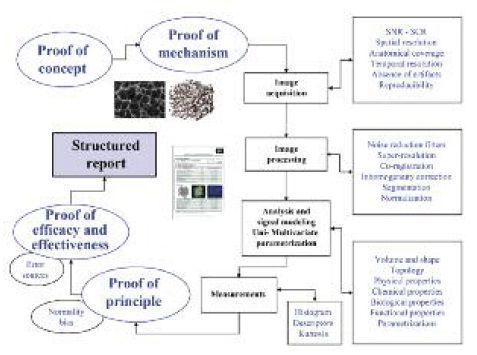
Although many research groups worldwide obtain several different indexes and parameters resulting from newly developed image processing algorithms, only a certain number of them will became clinically useful imaging biomarkers. Valid imaging biomarkers must give the answer to real biological and clinical problems; thus, they must be justified by clear proofs of concept and mechanism. The integration of new imaging biomarkers in clinical trials and patient evaluation is not straightforward and must meet strict conditions in terms of consistency, technical reproducibility, sensitivity and specificity. It is important to have a precise definition of the clinical problem to assess:
- Image acquisition procedures;
- Methods used in the analysis;
- Election of the proper computational models for biomarker extraction; and
- How the derived data is structured, reported and provided to the clinician.
Definition, development, expansion and implementation parts are the main segments of the innovation roadmap with imaging biomarkers.
Image Acquisition
With regards to acquisition, the gradual emergence of new generations of CT, MRI and also hybrid systems (i.e. PET-CT, PET-MR), have supposed a change of paradigm in medical imaging, since high amounts of new data are generated for each patient. For the success of imaging biomarkers’ innovation, optimised image acquisition procedures providing excellent image characteristics must be developed, accepted and standardised by the radiological community. The way images are acquired has a crucial impact in the quality of the data that will be evaluated. This is a highly relevant step to guarantee that the signal changes of the object of interest are related to the underlying biological alterations produced by the disease and not produced by the acquisition technique itself.
As in any other analytical procedure, it is also mandatory to perform periodic quality assurance and calibration evaluations in order to follow up on the stability and proper performance of the image acquisition equipments. Phantoms with different modules must be used to check that the variations in frequencies, signal-tonoise ratios (SNR), uniformity and spatial distortion are stable and within given confidence ranges.
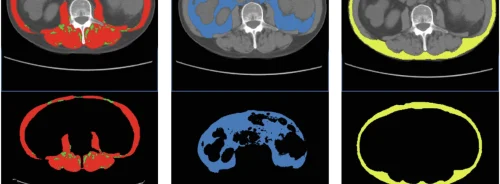
After image acquisition, several items for a proper data preparation and handling must be followed. Filters are one of the most used tools to minimise image noise. Also, interpolation algorithms can be applied for the maximisation of the spatial resolution and enhancing the detail of the images. An accurate spatial coherence between all the points of a tissue or organ during the entire study should be guaranteed by specific co-registration algorithms, which would help to ensure that a given area of an organ is represented by the same points in all the image series. Segmentation algorithms are also crucial since they allow tissue and organ isolation and classification, therefore limiting the image processing time to the tissue of interest. The most user-independent segmentation algorithms are preferred to minimise inter-subject variability of the methodology for the extraction of the imaging biomarker.
Data Analysis Methods
The methods for data analysis cannot be easily stratified due to the many procedures and characteristics that can be quantified. However, the most common are related to the assessment of volume and morphometry properties of structures, mechanical properties of tissues, metabolite concentrations, evaluation of new vessel formation (neoangiogenesis) and cell density (proliferation) quantification.
Imaging biomarkers can be measured and expressed in many different ways, from simple averages in a given region, parametric maps showing the regional distribution of a given indicator, and even multivariate parametric images for a more efficient assessment of the relevant clinical questions.
Implemention of Biomarkers in the Imaging Department
New imaging biomarkers will only have an impact in clinical practice if the information they provide answers the clinical/biological problem and the derived data is organised and exposed in a simple and intuitive way. Post-processing platforms with generation of integrated structured imaging biomarkers reports should be implemented and their communication skills tested. This would be the best way for a paradigm change in the radiological workflow in the radiology departments.
Some discrepancies may arise regarding the practical implementation of imaging biomarkers in most radiology departments. Generally speaking, there is a gap between the radiologist's daily heavy workload and certain time pressured technical issues as the control of image acquisition technology and its physical basis, the adequate interpretation of the information contained in images from different modalities, and the reproducibility and accuracy of imaging biomarker measurements. These reasons have motivated the progressive integration of biomedical engineers in the radiological scenario.
The adequate development, implementation and clinical incorporation of new imaging biomarkers would be the main role of biomedical engineers in the radiology department. Working together, radiologists and engineers may lead this personalised and precisionbased revolution in healthcare. If needed, it should be noted that for image processing and quantification, the physical presence of engineers in the radiology department is not mandatory. In fact, imaging biomarker analysis, once they are defined and the image acquisition is structured, can be outsourced to specialised image processing companies or groups.