There is a need for a cost-effective, quantitative imaging tool that can be deployed endoscopically to better detect early-stage gastrointestinal cancers, responsible for over a third of cancer-related deaths. Spatial frequency domain imaging (SFDI) is a low-cost imaging technique that produces near-real time, quantitative maps of absorption and reduced scattering coefficients, but most implementations are bulky and suitable only for use outside the body. Drs. Jane Crowley and George S. D. Gordon aimed to develop an ultra-miniature SFDI system comprising an optical fibre array, a micro camera, and a phase-tracking algorithm to rapidly extract images with fringe projections at three equispaced phase shifts to perform SFDI demodulation. Their promising results were recently published in the Journal of Biomedical Optics.
A novel approach to minimally invasiveendoscopic imaging
Gastrointestinal cancers comprise more than a third of global cancer cases and deaths. While population-based endoscopic screenings can reduce mortality rates, current diagnostic colonoscopies often (26%) miss certain types of polyps. There's a demand for an improved, minimally invasive contrast imaging device that can be used endoscopically. Such a device must have a diameter under 3mm to fit through a standard colonoscope's instrument channel and be cost-effective for widespread screening programmes. Spatial Frequency Domain Imaging (SFDI) offers a solution, providing quantitative maps of tissue properties in near real-time using a low-cost technique. It involves projecting a known 2D illumination pattern onto the sample, capturing the result with a CMOS camera, and performing demodulation to obtain modulation amplitudes for analysis.
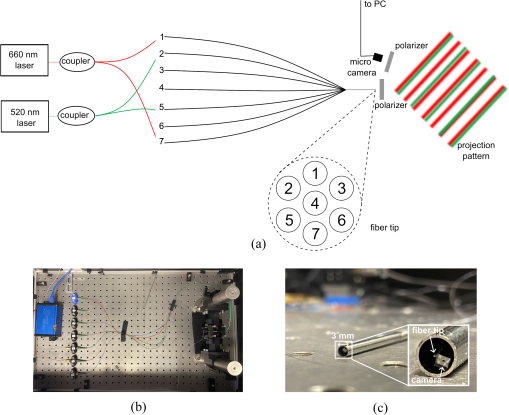
Image Source: SPIE
Promising projector performance
This innovation demonstrated its efficacy in quantitatively imaging differences in optical properties typical of gastrointestinal conditions, as simulated in tissue-mimicking phantoms. The expected spatial frequency of the projected illumination pattern is comparable to the desired spatial frequency, with 12% and 7% error for 660 and 515 nm, respectively. Some channels produce clearer interference patterns than others due to crosstalk between fibres. The system, small enough to fit into the instrument channel of a standard colonoscope at less than 3 mm in diameter, demonstrated its capability to detect quantifiable variances in tissue properties with errors comparable to conventional bench-top systems. By imaging variations in absorption and scattering coefficients, the system offers enhanced contrast between different tissue types, holding promise for the early detection of gastrointestinal cancers, in a cost-effective manner.
Further research needed before clinical translation
The study, however, acknowledges several limitations that require further investigation before clinical translation. Firstly, the choice of wavelengths, set at 660 and 515 nm in the experiments, raises considerations for optimal tissue analysis. While these wavelengths allow for the evaluation of important tissue properties such as chromophore concentration, future iterations may explore wavelengths more commonly used in tissue analysis, such as 670 and 850 nm.
Secondly, real-time operation for clinical application poses a challenge due to the time required to obtain suitable frames for SFDI. The study proposes solutions to enhance operational efficiency, including the utilization of advanced technologies like phase-shifters or spatial light modulators. Additionally, the implementation of faster processing algorithms, potentially on GPU platforms, could facilitate real-time operation.
Moreover, concerns regarding image quality arise from non-ideal illumination patterns generated by the fibre array, leading to noise and inaccuracies in optical properties. Strategies to mitigate these issues include normalisation for laser power, noise reduction techniques using artificial intelligence, and alternative calibration procedures.
Despite these limitations, the device held its promise, and further miniaturisation could explore advanced technologies such as metasurfaces for polarizers and various filters to enhance imaging capabilities. Overall, the study underscores the potential of the ultra-miniature SFDI system in revolutionising endoscopic screening for gastrointestinal cancers, offering a glimpse into the future of medical diagnostics.
Source: SPIE Digital Library
Image Credit: iStock