Lung cancer remains the most common cancer worldwide, with non-small cell lung cancer accounting for the majority of cases. Among these, lung adenocarcinoma is often characterised by epidermal growth factor receptor (EGFR) mutations, which are critical for guiding treatment with tyrosine kinase inhibitors. These targeted therapies can significantly extend survival but are only effective in patients with specific EGFR mutations. Current detection methods rely on tissue biopsies or liquid biopsies, which are invasive, costly and sometimes unreliable due to tumour heterogeneity or limited sensitivity. This has created demand for non-invasive approaches that are accurate, efficient and clinically applicable.
Radiomics and deep learning applied to CT imaging have demonstrated promise in assessing tumour biology, but most efforts have focused only on intratumoural regions or single modelling strategies. Recent research has examined whether including peritumoural regions and combining multiple analytical models can improve prediction accuracy. The findings show that integrating radiomics, deep learning and clinical features provides a more robust framework for non-invasive EGFR mutation prediction.
Radiomics and the Value of Peritumoural Regions
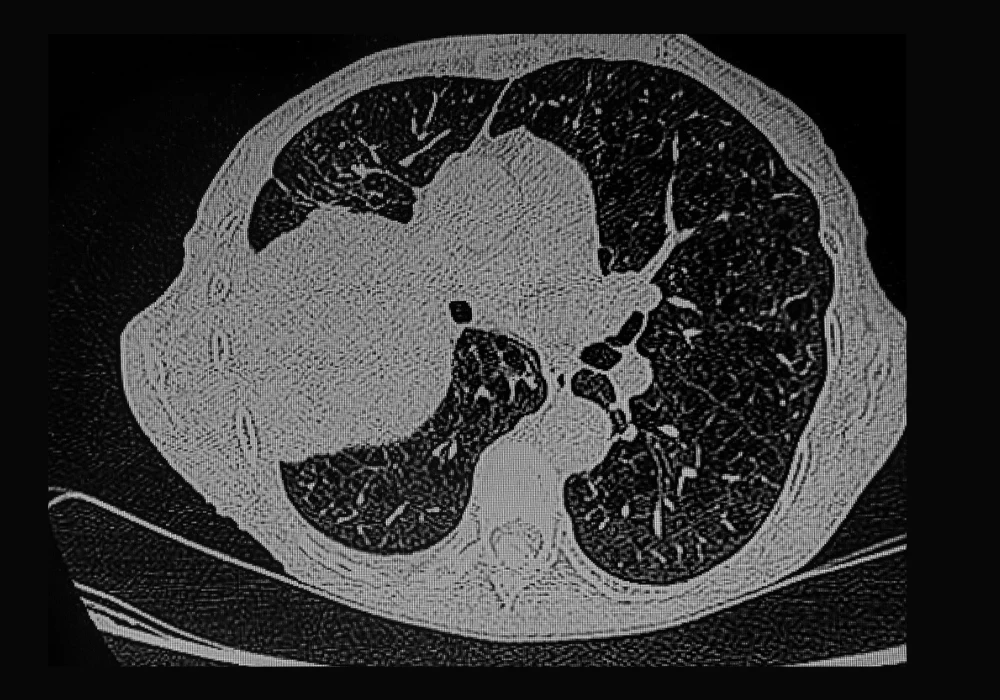
Radiomics involves extracting high-dimensional features from medical images to capture tumour heterogeneity, shape and texture. In the study in question, 826 lung adenocarcinoma patients were retrospectively analysed using thin-slice CT scans from two centres. Tumours and their surrounding regions were segmented with the aid of an automated nnUNet tool, which achieved high Dice coefficients, confirming robust performance. Radiomic features were extracted not only from tumour regions but also from concentric peritumoural zones at distances ranging from 2 to 10 millimetres.
Must Read: Non-Invasive Imaging for Lung Cancer Prediction
The results highlighted that incorporating peritumoural features enhanced predictive accuracy compared to models restricted to tumour-only regions. In particular, combining the tumour region with a 2 mm peritumoural zone achieved the highest performance, with area under the curve (AUC) values of 0.843 and 0.803 in internal and external validations. Shapley value analysis identified key radiomic parameters such as percentile intensity measures and texture correlations as influential predictors. These findings emphasise that biological changes in surrounding tissues provide critical signals that can support accurate classification of EGFR status.
Deep Learning Models Across 2D, 2.5D and 3D Spaces
In addition to radiomics, multiple deep learning networks were trained using two-dimensional, 2.5-dimensional and three-dimensional representations of tumours and peritumoural regions. The 2D models benefited from transfer learning with ImageNet data but were limited to single-layer images. The 2.5D models combined orthogonal planes to provide more context, while the 3D models processed entire volumetric data to capture complex spatial features.
Consistently, 3D networks outperformed 2D and 2.5D models across most regions, reflecting their ability to capture richer structural details. For the combined tumour and 2 mm peritumoural zone, the 3D deep learning model achieved an AUC of 0.814 in external validation, the highest among all deep learning models tested. Visualisation using Grad-CAM saliency maps confirmed that predictive attention extended beyond tumour borders into surrounding tissue, supporting the inclusion of peritumoural regions.
Interestingly, the relative advantage of 3D models diminished as the peritumoural region expanded beyond 6–10 mm, suggesting that overly broad regions may introduce noise. DenseNet-based architectures consistently showed the strongest performance, likely due to their efficient feature propagation and resistance to degradation. These results reinforce the need to balance regional coverage with signal relevance and computational demands in deep learning pipelines.
Fusion Strategies for Enhanced Prediction
While radiomics and deep learning each provide unique strengths, their integration with clinical features offers the most powerful predictive framework. Clinical variables such as age, sex and smoking status demonstrated moderate predictive value on their own, with AUCs of 0.703 and 0.649 for internal and external sets. However, by fusing clinical, radiomic and deep learning models, predictive performance improved significantly.
Different fusion strategies were evaluated, including early fusion at the feature level and late fusion through hard voting, soft voting and stacking. The soft voting approach achieved the best overall results, with AUCs of 0.925 and 0.889 in internal and external validations. These figures were statistically superior to those of any single modality model or alternative fusion methods. The weighting scheme in the soft voting strategy provided flexibility and interpretability, reducing overfitting risk while maintaining robust performance. Notably, the radiomics component contributed most strongly to predictions, with deep learning and clinical factors providing complementary gains.
This multimodal approach demonstrates that synergistic use of different feature domains can deliver non-invasive EGFR mutation prediction with high reliability. Importantly, these results were validated across two independent clinical centres, supporting the generalisability of the findings.
Accurate identification of EGFR mutations is central to guiding targeted therapy in lung adenocarcinoma. Traditional biopsy methods face challenges of invasiveness, cost and sampling limitations, creating a need for imaging-based alternatives. By analysing both intratumoural and peritumoural regions with radiomics and deep learning, and by fusing these with clinical features, prediction performance was markedly enhanced.
The findings highlight that peritumoural information captures biologically relevant changes beyond the tumour itself, that 3D deep learning models provide richer spatial insights, and that fusion strategies, particularly soft voting, offer the most reliable outcomes. Such approaches support the shift toward non-invasive, personalised treatment planning in lung cancer management.
Future work should expand to prospective studies, incorporate contrast-enhanced CT and stratify EGFR mutation subtypes to refine predictive capabilities further. The implications are clear: integrating advanced imaging analytics into diagnostic pathways can reduce dependence on invasive testing, optimise therapeutic decision-making and enhance patient-centred care in oncology.
Source: Academic Radiology
Image Credit: iStock