Artificial intelligence (AI) integration in healthcare has shown remarkable potential, particularly in the early detection of diseases such as breast cancer. Timely identification of cancer is crucial for improving survival rates and reducing the intensity of required treatment. A recent study conducted within the BreastScreen Norway programme examined the efficacy of a commercial AI algorithm not only in detecting existing cancer but also in estimating the risk of developing breast cancer years before diagnosis.
Evaluating the Study's Framework
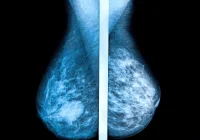
The study was structured as a retrospective cohort analysis involving 116,495 women aged 50 to 69. These participants had no previous history of breast cancer and had undergone at least three consecutive biennial screening rounds within BreastScreen Norway from 2004 to 2018. The screenings were conducted across nine breast centres using standard mammography procedures. The AI tool applied in this investigation was Lunit INSIGHT MMG, a commercially available algorithm that provides a continuous score indicating the likelihood of cancer presence. The scores range from 0 to 100, with higher numbers representing a greater probability of cancer. The study sought to evaluate whether the algorithm’s scores could effectively identify imaging features that indicate cancer development years in advance.
The analysis was stratified into three groups: women who developed screening-detected cancer by the third screening round, women diagnosed with interval cancer (detected between regular screenings) and women who showed no signs of cancer over the entire study period, including two additional years of follow-up. The primary outcome focused on AI scores from mammograms and the absolute difference in scores between the two breasts.
Key Findings on AI Efficacy
The study found distinct differences in AI scores between women who later developed cancer and those who did not. Notably, the mean AI score for breasts that eventually developed screening-detected cancer rose significantly across the three study rounds, from 19.2 in the first round to 82.7 in the third. For interval cancers, the scores were comparatively lower but still notable, indicating that the algorithm could recognise subtle pre-cancerous signs even when radiologists could not.
One of the most compelling findings was the rapid increase in the AI scores leading up to the third screening round for women who developed screening-detected cancer. This pattern suggests that AI can identify progressive changes in mammograms that precede clinical diagnosis. The AUC values, used to assess the discriminatory power of the AI tool, improved significantly by the third round, reaching 0.97 for screening-detected cancer and 0.78 for interval cancers. This demonstrates that the AI algorithm had high accuracy in distinguishing between breasts that would and would not develop cancer, especially close to the time of detection.
For women without a breast cancer diagnosis, the mean AI scores remained consistently low across all screening rounds, underscoring the tool's specificity. The absolute difference in AI scores between the breasts also provided valuable insight, with larger discrepancies observed in those developing cancer compared to those who did not.
Implications for Screening Practices
The findings from this study have substantial implications for how breast cancer screening might be approached in the future. The ability of an AI algorithm to indicate cancer risk up to six years before clinical detection could transform current screening protocols. Integrating such AI tools into regular mammography practices could help identify women at higher risk much earlier, enabling more frequent or supplemental imaging as a preventive strategy. This personalised approach would ensure that high-risk women receive closer monitoring, potentially leading to earlier intervention and less invasive treatment.
Moreover, using AI as an adjunct to radiologists could help prioritise which cases require further scrutiny. The data suggest that the absolute differences in AI scores between the breasts could serve as a valuable indicator of elevated risk. However, the study emphasised that while AI shows promise, it should be used in conjunction with human expertise to minimise false positives and unnecessary diagnostic procedures. This dual approach would ensure a balance between enhanced early detection and maintaining manageable screening recall rates.
The role of AI in streamlining radiologists' workloads cannot be overlooked. By helping to flag cases with higher risk scores, radiologists can focus their attention on cases that require more detailed analysis. This optimised workflow could contribute to more efficient screening processes and better allocation of healthcare resources.
The study conducted within the BreastScreen Norway programme underscores the transformative potential of AI in breast cancer screening. The results demonstrated that commercial AI algorithms can identify subtle imaging features years before a clinical diagnosis, offering a pathway for personalised and earlier intervention. By integrating AI into current screening practices, healthcare providers could improve the accuracy of early detection, leading to better patient outcomes. However, further research and careful implementation are essential to ensure that AI tools are used effectively and ethically, complementing radiologists' expertise and maintaining a low rate of false positives. The future of breast cancer screening looks promising, with AI playing a pivotal role in risk-based and proactive healthcare.
Source: JAMA Network Open
Image Credit: iStock