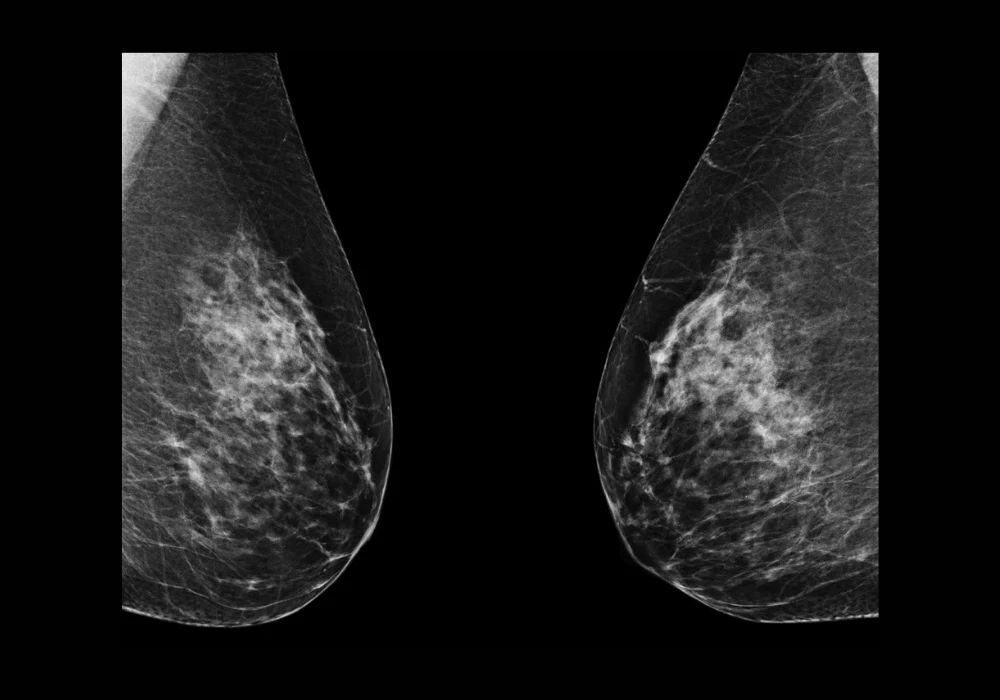
Breast arterial calcification (BAC), once considered a benign incidental finding in mammographic imaging, is now increasingly recognised as a significant marker of cardiovascular disease (CVD) risk. Its detection through standard mammograms offers a unique opportunity for dual-purpose screening in women, targeting both breast cancer and cardiovascular risk without added radiation or cost. Despite its potential, current BAC detection methods are hindered by variability and labour-intensity. A recent study introduced a novel deep learning model built on a modified U-Net architecture to automate the detection, segmentation and quantification of BAC in mammograms. The goal is to deliver a reliable tool that supports radiologists and facilitates further research into BAC’s prognostic value.
Deep Learning Framework for BAC Segmentation
To overcome the limitations of manual and conventional automated approaches, the study developed a tailored U-Net model enhanced with a composite loss function. This function integrates Dice loss, Binary Cross-Entropy (BCE) loss and, notably, Hausdorff loss, which strengthens the model’s ability to delineate fine calcification boundaries—an essential factor in accurate BAC segmentation. The researchers trained and validated their model using a diverse dataset of mammograms from 369 women aged 60–79, acquired from multiple vendors and imaging views. Manual annotations by trained readers ensured quality ground truth data. Pre-processing steps included standardising image resolution, applying data augmentation and cropping to focus on breast tissue. This robust setup enabled the model to retain image fidelity and learn orientation-invariant features critical for generalisability across real-world scenarios.
The segmentation results demonstrated high accuracy, with Dice similarity coefficients reaching 0.90 for the training set and 0.88 for the validation set. The model’s performance surpassed previous techniques that relied on downscaled or patch-based input data. An ablation study confirmed that the inclusion of Hausdorff loss significantly improved boundary delineation, especially in complex tubular BAC structures. This advancement marked a critical step toward achieving precise segmentation essential for accurate mass quantification.
Accurate Quantification and Model Validation
Beyond segmentation, the study implemented a densitometric interpolation method to quantify BAC mass, allowing for clinically meaningful stratification. This method calculates the mass of calcified deposits by comparing signal intensity within segmented regions to background levels, using calibration data derived from imaging phantoms. Stratified BAC mass ranges, such as 0–20 mg and >80 mg, were used to reflect varying degrees of cardiovascular risk. Bland–Altman analysis and linear regression confirmed the model’s strong agreement with manual quantification, with minimal bias and high reproducibility. For instance, the model achieved R² values of 0.99 for the training set and 0.98 for the validation set.
Must Read: Exploring the Link Between Breast Density and Cardiovascular Risk
Further comparison with a previously established deep learning method showed the superiority of the new model. On a shared dataset of 113 patients, the proposed method demonstrated lower root mean square error (2.3 mg vs. 5.35 mg) and better segmentation precision, thanks to its use of the full-resolution images and advanced post-processing techniques. These improvements suggest that the model not only enhances predictive accuracy but also reduces observer variability, making it a promising tool for large-scale clinical application.
Implications for Dual-Purpose Screening
The high classification accuracy (F1 score of 0.96 in the validation set) reinforces the model’s potential for distinguishing between BAC-positive and BAC-negative cases. This capability supports the integration of BAC screening into routine mammography workflows, enabling simultaneous assessment of breast cancer and cardiovascular risk. Such integration could prove particularly beneficial for women with borderline cardiovascular risk profiles, offering an accessible and non-invasive way to monitor subclinical disease progression.
In practical terms, the model could be embedded within existing PACS environments, delivering real-time analysis as images are acquired. Results could be incorporated into standardised reports alongside breast cancer findings, improving efficiency and cost-effectiveness. However, widespread implementation would require standardised BAC scoring systems and clear clinical guidelines for cardiovascular referrals based on quantified BAC mass. While this study focused on digital mammograms, future research will need to assess the model’s performance with synthetic mammography and explore the role of breast density in BAC detection accuracy.
The study demonstrated that a carefully designed deep learning framework can significantly enhance the detection, segmentation and quantification of BAC in mammograms. By combining full-resolution image analysis, a novel composite loss function and a validated quantification technique, the model achieves a high level of accuracy and reliability. These advancements pave the way for mammography to evolve into a dual-purpose screening modality, addressing both breast cancer and cardiovascular risk. Future research should focus on integrating such tools into clinical practice, refining risk stratification protocols and exploring the broader epidemiological implications of BAC in women’s health.
Source: Academic Radiology
Image Credit: iStock