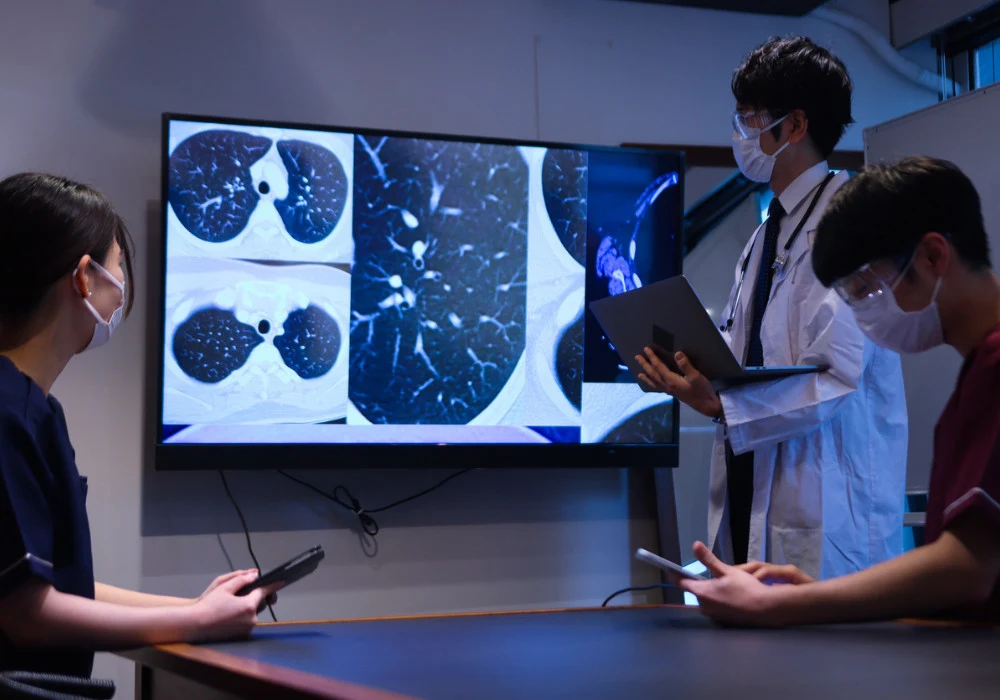
Chronic obstructive pulmonary disease is heterogeneous, with emphysema, chronic bronchitis and recurrent exacerbations driving morbidity. Global cases among adults are projected to rise by 23% from 2020 to 2050, approaching 600 million, underscoring the need for tools that characterise disease beyond spirometry. Chest CT has become central to quantifying structural and functional abnormalities, with large observational cohorts acquiring imaging alongside clinical and biological data. A growing suite of CT biomarkers now spans parenchyma, airways, vasculature, fissures and mucus, complemented by image registration, radiomics and deep learning methods. Together they aim to refine subphenotypes, track progression and support more personalised COPD management, while ongoing work addresses protocol variability, generalisability and interpretability.
Parenchymal and Texture Biomarkers
Early CT approaches quantified global lung density and volumes, then progressed to density mask analyses on inspiratory and expiratory scans to segment parenchyma by Hounsfield thresholds. Measures such as the emphysema index, Perc15 and adjusted lung density capture parenchymal destruction, while expiratory thresholds quantify air trapping as a marker of small airway dysfunction. These metrics provide a structured view of severity and progression by linking attenuation distributions to tissue loss and ventilation heterogeneity.
Because thresholding overlooks spatial organisation, texture analysis emerged to characterise clustering and distribution of abnormalities. Supervised, semisupervised and unsupervised methods have been used to differentiate patterns across the lung, from emphysema-like regions to ground glass, reticular change and normal tissue. Notably, adaptive multiple feature methods in 2D and 3D generated repeatable texture maps and were later related to outcomes including exacerbations and mortality. Radiomics extended this concept with first-order, shape and matrix-based texture features that quantify homogeneity, contrast and complexity. More recently, deep learning has automated feature extraction directly from raw images, improving precision in phenotype characterisation and risk prediction by learning hierarchical representations of tissue heterogeneity.
These parenchymal descriptors continue to evolve. Quantitative CT textures have been associated with systemic inflammation and mortality, linking imaging to biological pathways. As datasets broaden, texture and radiomics signatures are being used to define subtypes with distinct characteristics and prognoses, supporting stratification that complements spirometric classification.
Airway, Vascular and Structural Metrics
Airway-focused biomarkers have advanced from threshold-based detection of large bronchi to quantitative assessments of wall geometry and branching architecture. Parameters such as wall area percentage, standardised wall thickness metrics and lumen area quantify remodelling and obstruction. Surface area to volume ratios and fractal dimensions capture narrowing and loss of complexity, while newer measures like PiSlope and airway tapering assess non-uniform wall change and cumulative cross-sectional area along the airway tree, offering specificity for progressive remodelling. These measures reflect the central role of airway inflammation and structural change in COPD pathogenesis.
Must Read: CT-Based Multimodal Analysis for EGFR in Lung Cancer
Vascular biomarkers derived from CT add a complementary perspective. Total intraparenchymal blood vessel volume and the small-vessel fraction below 5 mm² reflect vascular pruning and correlate with airflow limitation, emphysema and oxygenation impairment. The pulmonary artery to aorta diameter ratio has emerged as a predictor of exacerbation risk and pulmonary hypertension, and right ventricular volume measured on CT signals right heart dysfunction in advanced disease. Some mediastinal metrics require contrast-enhanced scans to delineate vessels accurately, a consideration for protocol planning and longitudinal follow-up.
Structural segmentation of pulmonary fissures has gained importance because fissure completeness relates to disease severity, collateral ventilation and response to volume reduction interventions. While traditional edge and atlas methods struggled with poor contrast and anatomical variability, deep learning approaches such as FissureNet improved delineation across diverse patients, enabling more reliable integration of lobar anatomy into phenotyping.
Mucus plugging represents another airway phenotype with prognostic weight. Using segment-level assessment on CT, airway-occluding plugs in medium to large airways were associated with increased all-cause mortality over long follow-up, even after accounting for comorbidities and substituting gas trapping for emphysema in models. Emerging automated methods now detect and count plugs, opening pathways to reproducible analysis at scale and targeted therapies that address mucus burden.
Registration, Multiomics and Emerging AI
Image registration of inspiratory and expiratory scans enables spatially aware biomechanics. Voxel-wise Jacobian determinants quantify local tissue expansion and contraction, and variations in these metrics correlate with disease severity and clinically relevant outcomes including lung function, respiratory morbidity, exercise capacity and mortality. Mechanically affected regions have been linked to faster emphysema progression and greater risk of decline, highlighting the value of biomechanical maps for risk assessment.
Registration also underpins parametric response mapping, which differentiates functional small airway disease from emphysema by classifying voxels according to attenuation changes between respiratory phases. A higher burden of functional small airway disease associates with accelerated lung function decline, increased symptoms and greater exacerbation risk. Disease probability maps provide another registration-based view by representing voxel-wise likelihood of abnormality from displacement fields, supporting spatial assessment of early disease.
Multiomics integration is aligning imaging phenotypes with molecular signatures. Associations have been shown between CT textures and inflammatory biomarkers, and between emphysema measures and soluble receptors for advanced glycation end products. These connections position imaging features as intermediate phenotypes that reflect underlying pathways such as inflammation and tissue remodelling, strengthening prospects for targeted interventions informed by both imaging and biology.
Deep learning continues to expand the toolkit. Generative and transformer-based models have been developed to identify textures most susceptible to emphysema progression and to automate mucus plug quantification. Local surrogates of lung function can now be estimated from single-volume CT using generative adversarial and residual regression networks, with single-scan estimation of functional small airway disease validated across cohorts and associated with decline, morbidity and mortality. These advances promise faster, more standardised analysis in routine workflows.
Important constraints remain. Variability in scanner type, acquisition parameters, reconstruction methods and dose affects biomarker reliability, particularly in multicentre studies. Population heterogeneity and radiation exposure considerations also influence adoption, despite progress with low-dose protocols. Hardware innovation such as photon-counting CT offers improved resolution, contrast and noise reduction at lower dose, while foundation models trained on diverse datasets aim to improve robustness across scanners and populations.
Chest CT now provides a comprehensive framework for COPD phenotyping that spans parenchymal density, texture and radiomics, detailed airway and vascular metrics, fissure integrity and mucus burden, together with biomechanical mapping from image registration. Integrated with multiomics and accelerated by deep learning, these biomarkers support refined subtypes and risk prediction beyond spirometry. Realising their full clinical value will depend on harmonised protocols, validation across populations and continued advances in dose-efficient imaging and robust models to ensure reproducible, interpretable outputs that inform personalised care.
Source: British Institute of Radiology
Image Credit: iStock