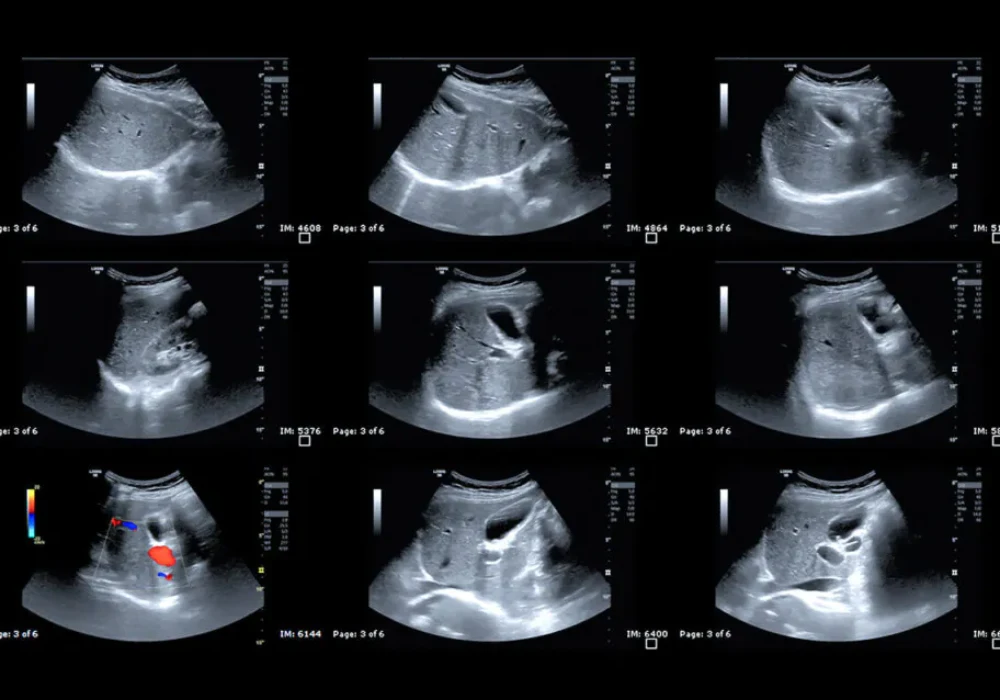
Knowing how aggressive a liver tumour is before treatment can shape decisions on surgery and follow-up. Pathology remains the benchmark for confirming tumour behaviour, yet it becomes available only after resection. Contrast-enhanced ultrasound (CEUS) provides a real-time view of blood flow in and around hepatocellular carcinoma, but visual interpretation can vary between readers. An analysis explored whether turning CEUS video into objective numbers can improve preoperative recognition of two aggressive patterns, macrotrabecular-massive subtype and a high Ki-67 pattern, by combining these measurements with routine clinical and imaging features across a development cohort and an external validation cohort.
Cohorts, Imaging Workflow and Definitions
The work drew on 170 surgically resected hepatocellular carcinomas from a retrospective primary cohort and a prospective two-centre validation cohort. Patients underwent preoperative CEUS using SonoVue and examinations were categorised with CEUS LI-RADS to structure interpretation. Pathology after surgery served as the reference standard. Macrotrabecular-massive subtype was defined by thick trabeculae occupying more than half of the tumour area, while a high Ki-67 pattern meant more than 20% of cells stained positive on immunohistochemistry. In patients with multiple lesions, the largest was selected for analysis.
Must Read: Synthetic CEUS for Tumour Ablation Assessment
Ultrasound and CEUS images were read independently by two radiologists with more than five years of experience, with a senior radiologist resolving disagreements. Agreement within and between readers ranged from moderate to excellent. To reduce subjectivity, CEUS clips were processed offline using software that calibrated the signal, applied motion compensation and extracted time–intensity curves from regions of interest positioned over the lesion, the immediate margin, surrounding liver and a broader border area. Only curves with adequate fit quality were used. From these curves, the software calculated a suite of perfusion metrics, including average contrast signal intensity known as MeanLin, peak enhancement, wash-in and wash-out areas under the curve, wash-in rate, wash-in perfusion index and wash-out rate.
Predictive Features and Added Value of Quantification
Clinical variables and conventional imaging signs associated with aggressive biology were recorded. Macrotrabecular-massive tumours were more often linked to higher preoperative α-fetoprotein, larger size, higher histological grade and both macrovascular and microvascular invasion than non-macrotrabecular-massive disease. On ultrasound and CEUS, they more frequently showed size greater than 5 cm, intratumoural necrosis, intratumoural arteries, peritumoural nutrient vessels, heterogeneous arterial enhancement, branched arterial enhancement and hypo or marked hypo-enhancement in the portal venous phase. High Ki-67 tumours were associated with younger age, higher α-fetoprotein, higher AFP-L3%, larger size, higher grade and microvascular invasion, and they more commonly displayed intratumoural necrosis and branched arterial enhancement.
Quantitative CEUS provided objective distinctions beyond visual features. Compared with non-macrotrabecular-massive tumours, the macrotrabecular-massive subtype had higher lesion and margin MeanLin ratios, together with higher margin peak enhancement, wash-in and wash-out areas under the curve and their sum. Tumours with high Ki-67 showed higher lesion MeanLin, lesion peak enhancement and lesion wash-in perfusion index ratios, plus multiple elevated margin metrics including MeanLin, peak enhancement, wash-in area, wash-in rate, wash-in perfusion index and wash-out rate. These patterns indicate that lesion brightness and margin perfusion behaviour captured by CEUS numbers can signal aggressive phenotypes before pathology is available.
Two multivariable frameworks were developed to turn these observations into practical prediction tools. A Clinic-CEUS model combined clinical data with conventional ultrasound and CEUS features. A Clinic-Q-CEUS model added dynamic quantitative parameters from the software analysis. For the macrotrabecular-massive subtype, independent predictors were α-fetoprotein greater than 20 ng/mL, portal venous phase hypo or marked hypo-enhancement, lesion MeanLin ratio at least 3.96 and margin MeanLin ratio at least 1.43. For the high Ki-67 pattern, independent predictors were age under 59 years, AFP-L3% greater than 10 U/mL, lesion MeanLin ratio at least 2.98 and margin wash-in perfusion index ratio at least 2.55.
Model Performance in Development and Validation
Adding quantitative CEUS strengthened discrimination in both cohorts. In the primary cohort, the area under the receiver operating characteristic curve rose from 0.753 with the Clinic-CEUS model to 0.860 with the Clinic-Q-CEUS model for identifying the macrotrabecular-massive subtype. For recognising a high Ki-67 pattern, the area under the curve increased from 0.738 to 0.836 with the addition of quantitative parameters. External validation reproduced these gains, with the area under the curve increasing from 0.693 to 0.868 for the macrotrabecular-massive subtype and from 0.610 to 0.787 for the high Ki-67 pattern.
These improvements were accompanied by directionally consistent changes in accuracy and sensitivity, supporting the practical value of augmenting routine assessment with objective perfusion metrics. The results suggest that numbers derived from CEUS can reduce reader variability inherent to purely visual interpretation. In everyday terms, combining basic clinical information with standard imaging signs and a handful of robust CEUS ratios yielded models that were better at flagging tumours with adverse features before the surgeon operates.
Turning CEUS into numbers provided meaningful preoperative signals of aggressive hepatocellular carcinoma when combined with routine clinical and imaging data. Lesion and margin MeanLin ratios were informative for the macrotrabecular-massive subtype, while lesion MeanLin and margin wash-in perfusion index ratios supported recognition of a high Ki-67 pattern. Across development and external validation cohorts, models that included these quantitative parameters achieved higher discrimination than those relying on clinical and visual features alone. For healthcare teams, integrating CEUS quantification into reporting offers a practical way to strengthen risk stratification, refine surgical planning and focus follow-up without introducing additional invasive steps.
Source: Insights into Imaging
Image Credit: iStock