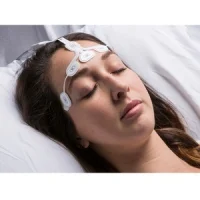
O3 regional oximetry uses near-infrared spectroscopy (NIRS) to continuously monitor absolute and trended regional tissue oxygen saturation (rSO2) in the cerebral region. Early detection and correction of imbalances in oxygen delivery to the brain are important tools in helping patients avoid postoperative morbidity and adverse outcomes.1
“O3 regional oximetry provides access to valuable data about cerebral oxygen saturation,” said Joe Kiani, Founder and CEO of Masimo. “With adult and pediatric trend accuracy of 3% and absolute accuracy of 4% and 5% on adults and pediatrics respectively,2 without controlling CO2, Masimo O3 should help clinicians build a better picture of brain oxygenation – and hopefully better outcomes for all of their patients, including pediatrics as young as three-months old.2”
In addition, Masimo O3 regional oximetry and SedLine® brain function monitoring are both available on a single platform, Masimo Root® – opening up a path to better understanding of the brain.
References | ||
| 1. | Booth, Dukatz, Ausman, and Wider. “Cerebral and somatic venous oximetry in adults and infants.” Surg. Neurol Int. 2010; 1: 75. | |
| 2. | Masimo data on file. | |