Reliable hygiene is currently a topic that shapes every aspect of our daily lives. More than ever before, the successful fight against dangerous viruses and multi-resistant germs in hospitals and other healthcare facilities is particularly important. High occupancy rates and resulting staff shortages are amplifying the challenge.
However, hospital acquired infections (HAI), or health care-associated infections (HCAI), have been the most frequent adverse event in the delivery of healthcare worldwide for many years now. A fact sheet on the HCAI endemic burden, published by the World Health Organisation (WHO), states that “of every 100 hospitalized patients at any given time, 7 in developed and 10 in developing countries will acquire at least one health care-associated infection.”1 The risk is even higher for patients in the ICU. Here, around 30% of patients show at least one hospital acquired infection.

Hospital beds need thorough disinfection
There are a lot of different factors that put patients at risk of HAI, including underlying health conditions, bed occupancy and staff shortage among others. Another important factor is the facility’s hygiene and infection control protocol. Studies, for example, have shown that enhanced cleaning can significantly reduce patient acquisition of MRSA by more than 50%.2 And this is the task where modern hospital beds can actively support the fight against HAI. The cleaning and disinfection of beds plays an important role in hospitals. Beds are the centre of a patient’s hospital stay and are touched often not only by the patient but also by different staff and visitors alike. The hospital bed should therefore be cleaned thoroughly after every discharge or transfer of a patient. According to the Organisation for Economic Cooperation and Development (OECD) “the average length of stay in hospitals was slightly less than 8 days across OECD countries” in 2017.3 Therefore, a hospital bed should be cleaned and disinfected about 50 times per year. In addition, the German Robert Koch Institute highlights the “necessity to always guarantee that the tried and tested methods are of a constantly high and verifiable quality”.4
However, how do you guarantee that, for example, 200 hospital beds are properly cleaned and disinfected with the same high quality 50 times a year? That is a total of 10,000 reprocessing cycles within 365 days. The surest way to establish a high and verifiable quality is by using automated reprocessing with a suitable washing system for large hospital equipment such as beds. A set and validated process for hospital beds then assures that the quality remains the same across all reprocessing cycles. However, the hospital bed, the materials and electronic components used, have to be suited for automated reprocessing. The European DIN standard EN 60601-2-52 on the particular requirements for the basic safety and essential performance of medical beds demands durability over 50 wash cycles.
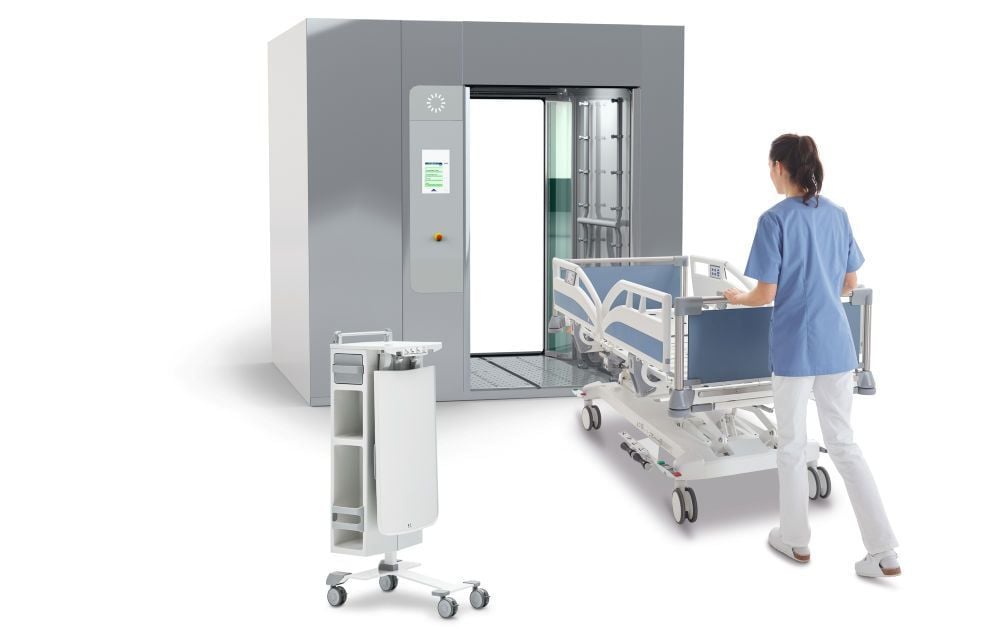
Stiegelmeyer hospital beds subjected to extensive washing tests
At Stiegelmeyer, the quality department tests the manufactured machine-washable hospital beds with several hundred cycles. The goal is to achieve the longest possible lifespan for these beds with maximum hygiene. Stiegelmeyer has its own washing system from Swiss manufacturer Belimed at its development centre in Germany – for the validation and verification of the washing tests. Time intervals, temperatures and chemicals are defined for each step of the washing cycle and can then be called up at the touch of a button once the bed is inside the washing system. With this validated process, Stiegelmeyer’s hospital bed models are subjected to extensive durability and usability washing tests. In this process, the bed is not only reprocessed in the washing system, but also tested with weights and adjustment cycles of the mattress base between washes to assure its resilience in everyday use.
Machine-washable Stiegelmeyer hospital beds have been distinguished by quality and durability for decades. An innovative paint formula protects the metal parts from corrosion. In addition, an effective cavity sealing is carried out before the powder coating. Of course, the paint is environmentally friendly and, depending on the bed selected, contributes to its attractive appearance in the colours White and Argentum. After hundreds of washes, customers can rely on the impeccable condition of these beds if they follow the operating instructions for the beds and the washing system. This has been confirmed by numerous tests in the Stiegelmeyer development centre in Germany. This high and valid quality of hygiene protects patients and staff.

Manual cleaning as an alternative to automated reprocessing
While the trend towards automated reprocessing is increasing, most hospitals still rely on cleaning and disinfection by hand. Products from Stiegelmeyer such as the Puro, Evario and Seta pro beds or the lightweight Quado bedside cabinet also offer strong advantages in manual reprocessing:
- Good handling characteristics when the way to the reprocessing area leads through narrow corridors and lifts
- Products are tested for resistance to disinfectants (recommendations’ list included in the bed's user manual)
- Large smooth surfaces are easy to clean
- Construction with few crevices and gaps where persistent dirt could get embedded
- Concealed cable routing
- Durability due to excellent workmanship
Whether your hospital is reprocessing its beds by hand or by automated washing system, you can rest assured that Stiegelmeyer beds are up for the task.
References:
World Health Organisation. (2011). Health care-associated infections [Fact sheet]. Retrieved from: https://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf?ua=1
Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665-690. doi:10.1128/CMR.00020-14
OECD (2019), Health at a Glance 2019: OECD Indicators, OECD Publishing, Paris, https://doi.org/10.1787/4dd50c09-en.
Commission for Hospital Hygiene and Infection Prevention (KRINKO); Federal Institute for Drugs and Medical Devices (BfArM). Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI) und des Bundesinstitutes für Arzneimittel und Medizinprodukte (BfArM) [Hygiene requirements for the reprocessing of medical devices. Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012 Oct;55(10):1244-310. German. doi: 10.1007/s00103-012-1548-6. PMID: 23011095. English text:







