A new study led by researchers at the Perelman School of Medicine at the University of Pennsylvania has found an aspect of chronic kidney disease (CKD) development that points to a promising new therapeutic strategy. The findings have been published online in advance of the print edition of Nature Medicine.
“We found that a defect in energy production in affected kidney cells plays a key role in CKD development,” said Katalin Susztak, MD, PhD, an associate professor of Medicine in the Renal Electrolyte and Hypertension Division. “Restoring the energy supply in these cells largely prevented signs of CKD in mouse models.”
CKD affects at least one in four Americans who are older than 60 and can significantly shorten lifespans. However, the few available drugs for CKD can only modestly delay the disease’s progression towards kidney failure, the researchers noted.
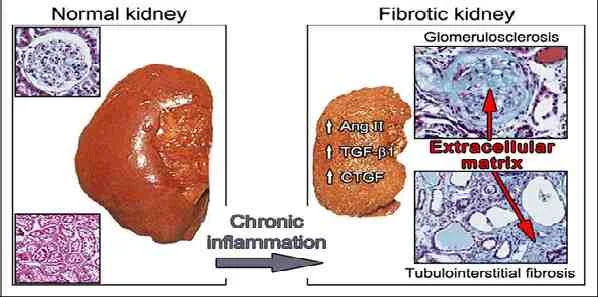
Dr. Susztak's study focused on a central feature of CKD: the “fibrosis” process. This is a pathological response to chronic kidney stress that includes an abnormal buildup of fibrous collagen, a die-off of important kidney cells called tubular epithelial cells, a loss of capillaries, and other changes that progressively reduce a kidney’s ability to filter the blood properly.
Dr. Susztak and colleagues compared the patterns of gene activity in fibrotic and normal human kidney tissue samples. They discovered abnormal patterns in gene networks linked to inflammation and sharp drops in activity in gene networks that support energy metabolism in the fibrotic samples.
It is already well known that inflammation is a factor in CKD. Dr. Susztak's team, therefore, aimed their investigation at two types of energy metabolism — glucose oxidation and fatty acid oxidation — that appeared markedly reduced in the fibrotic samples. They reported these interesting results:
“What we found is that the tubular pithelial cells preferentially use fatty acid oxidation as their energy source in normal conditions,” Dr. Susztak said. “Even when fatty acid metabolism drops in the context of CKD, these cells don’t switch to burning glucose for energy.” Hence, the more important factor in fibrosis was the loss of energy in the cells as fatty acid metabolism dropped.
In addition, Dr. Susztak et al. found evidence that the shutdown of fatty acid metabolism in tubular epithelial cells is caused in large part by the growth factor TGFβ. This factor is known to promote fibrosis and has been linked to high blood glucose levels, high blood pressure, and inflammation — all triggers of CKD.
Notably, when the researchers restored fatty acid metabolism in mouse models of kidney fibrosis using genetic techniques or compounds that boost the activity of fatty acid metabolism genes, the treatment prevented nearly all signs of fibrosis.
One of the compounds tested, fenofibrate, is an existing anti-cholesterol drug that activates a master switch gene, PPARA, for fatty acid metabolism. However, as Dr. Susztak noted, fenofibrate could be problematic as a kidney disease drug because it can distort the results of a standard test of kidney function. This side effect could impede the ability to evaluate the drug’s benefit, and could make it harder for clinicians to monitor the progression of CKD in patients taking the drug.
“We hope to develop new compounds that are similar to fenofibrate, or that boost enzymes more specifically related to fatty acid metabolism,” Dr. Susztak said. “In that way we might be able to greatly slow the progress of CKD.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK087635, R01DK076077) and the Diabetic Complications Consortium (DK076169).
Source: Newswise.com
Image Credit: Pharma Zentrum Frankfurt
“We found that a defect in energy production in affected kidney cells plays a key role in CKD development,” said Katalin Susztak, MD, PhD, an associate professor of Medicine in the Renal Electrolyte and Hypertension Division. “Restoring the energy supply in these cells largely prevented signs of CKD in mouse models.”
CKD affects at least one in four Americans who are older than 60 and can significantly shorten lifespans. However, the few available drugs for CKD can only modestly delay the disease’s progression towards kidney failure, the researchers noted.
Dr. Susztak's study focused on a central feature of CKD: the “fibrosis” process. This is a pathological response to chronic kidney stress that includes an abnormal buildup of fibrous collagen, a die-off of important kidney cells called tubular epithelial cells, a loss of capillaries, and other changes that progressively reduce a kidney’s ability to filter the blood properly.
Dr. Susztak and colleagues compared the patterns of gene activity in fibrotic and normal human kidney tissue samples. They discovered abnormal patterns in gene networks linked to inflammation and sharp drops in activity in gene networks that support energy metabolism in the fibrotic samples.
It is already well known that inflammation is a factor in CKD. Dr. Susztak's team, therefore, aimed their investigation at two types of energy metabolism — glucose oxidation and fatty acid oxidation — that appeared markedly reduced in the fibrotic samples. They reported these interesting results:
- After examining several mouse models of kidney fibrosis, the team again found strikingly lower activity in genes that support fatty acid metabolism.
- The team also found strong hints that the loss of cellular fuel is a driver of the fibrosis process.
- In mouse models, the drop in fatty acid metabolism preceded the signs of fibrosis.
- In human tubular epithelial cells, artificially reducing fatty acid metabolism quickly brought about fibrosis-like signs, including the buildup of fat molecules (unspent fuel) and the deaths of many affected cells.
“What we found is that the tubular pithelial cells preferentially use fatty acid oxidation as their energy source in normal conditions,” Dr. Susztak said. “Even when fatty acid metabolism drops in the context of CKD, these cells don’t switch to burning glucose for energy.” Hence, the more important factor in fibrosis was the loss of energy in the cells as fatty acid metabolism dropped.
In addition, Dr. Susztak et al. found evidence that the shutdown of fatty acid metabolism in tubular epithelial cells is caused in large part by the growth factor TGFβ. This factor is known to promote fibrosis and has been linked to high blood glucose levels, high blood pressure, and inflammation — all triggers of CKD.
Notably, when the researchers restored fatty acid metabolism in mouse models of kidney fibrosis using genetic techniques or compounds that boost the activity of fatty acid metabolism genes, the treatment prevented nearly all signs of fibrosis.
One of the compounds tested, fenofibrate, is an existing anti-cholesterol drug that activates a master switch gene, PPARA, for fatty acid metabolism. However, as Dr. Susztak noted, fenofibrate could be problematic as a kidney disease drug because it can distort the results of a standard test of kidney function. This side effect could impede the ability to evaluate the drug’s benefit, and could make it harder for clinicians to monitor the progression of CKD in patients taking the drug.
“We hope to develop new compounds that are similar to fenofibrate, or that boost enzymes more specifically related to fatty acid metabolism,” Dr. Susztak said. “In that way we might be able to greatly slow the progress of CKD.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK087635, R01DK076077) and the Diabetic Complications Consortium (DK076169).
Source: Newswise.com
Image Credit: Pharma Zentrum Frankfurt
Latest Articles
kidney failure, CKD, chronic kidney disease, fibrosis, glucose, fatty acid
A new study led by researchers at the Perelman School of Medicine at the University of Pennsylvania has found an aspect of chronic kidney disease (CKD) dev...