A medical imaging technology under development that enabled doctors to see bacteria deep inside patients' lungs for the first time was one of the highlights of ISICEM 2018.
A molecular imaging system under development by the PROTEUS consortium has enabled doctors to see bacteria deep inside the distal lung for the first time. Kev Dhaliwal, Professor of Molecular Imaging and Healthcare Technology at the University of Edinburgh updated delegates at the International Symposium on Intensive Care and Emergency Medicine in Brussels last week.
When a patient on mechanical ventilation deteriorates, rapid diagnosis is needed. However, it’s challenging to move them and few discriminatory tests exist. Often it is hard to see what a pulmonary infiltrate is on a chest x-ray, for example. What if we could see inflammation, bacteria and scarring in the lungs in seconds? What if we could measure physiology in the distal lung? The bedside molecular optical imaging and sensing platform under development will entail going deep into alveolar spaces, visualising in real time, and having punch information biopsies without taking tissue.
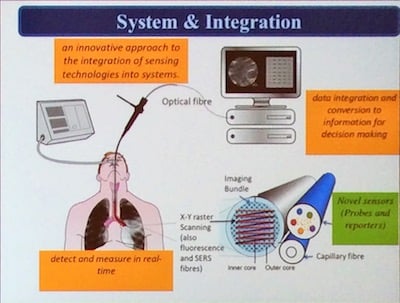
The smart probes (like molecular light bulbs, that go on when they hit the target) can be sprayed into patients’ lungs and have light emitting diodes that can suck tiny amounts of fluid out and also deliver imaging agents. They light up when they attach to specific types of infectious bacteria. This fluorescence is detected using fibre-optic tubes that are small enough to travel deep inside patients' lungs. The automated bronchoscopes have the ability to pass these fibres (imaging bundle, sensing bundle and capillary bundles) deep into the lung, combined with machine learning to understand signals and noise, which together is called molecular alveoscopy. The image analysis works by automated frame enrichment plus bacterial feature detection and cellular infiltration. It is computationally ‘light’ to enable real-time processing.
The ultimate optical molecular biopsy and optical fingerprint needs to be immediate (work within 60 seconds), usable at the bedside, cheap, safe, accurate and add to decision making, said Dhaliwal. It should be automated and self-guided to different regions of the lung with clear sampling and external registration, with the ability to sense, image and sample, and part of routine care just like a tracheal suction catheter. The probes need to be cheap, specific, scalable and pharmaceutical grade. One application is neutrophil imaging and a probe has been developed that binds neutrophils. Neutrophil mediated lung injury is an important pathological process in numerous conditions and especially in ARDS.
Dhaliwal emphasised the importance of the multidisciplinary research team for this project, which includes together engineers, physical scientists and chemists.

To sense, perturb and understand in vivo in situ process in human lungs is a long journey, but one we must take, concluded Dhaliwal.
A molecular imaging system under development by the PROTEUS consortium has enabled doctors to see bacteria deep inside the distal lung for the first time. Kev Dhaliwal, Professor of Molecular Imaging and Healthcare Technology at the University of Edinburgh updated delegates at the International Symposium on Intensive Care and Emergency Medicine in Brussels last week.
When a patient on mechanical ventilation deteriorates, rapid diagnosis is needed. However, it’s challenging to move them and few discriminatory tests exist. Often it is hard to see what a pulmonary infiltrate is on a chest x-ray, for example. What if we could see inflammation, bacteria and scarring in the lungs in seconds? What if we could measure physiology in the distal lung? The bedside molecular optical imaging and sensing platform under development will entail going deep into alveolar spaces, visualising in real time, and having punch information biopsies without taking tissue.

The smart probes (like molecular light bulbs, that go on when they hit the target) can be sprayed into patients’ lungs and have light emitting diodes that can suck tiny amounts of fluid out and also deliver imaging agents. They light up when they attach to specific types of infectious bacteria. This fluorescence is detected using fibre-optic tubes that are small enough to travel deep inside patients' lungs. The automated bronchoscopes have the ability to pass these fibres (imaging bundle, sensing bundle and capillary bundles) deep into the lung, combined with machine learning to understand signals and noise, which together is called molecular alveoscopy. The image analysis works by automated frame enrichment plus bacterial feature detection and cellular infiltration. It is computationally ‘light’ to enable real-time processing.
The ultimate optical molecular biopsy and optical fingerprint needs to be immediate (work within 60 seconds), usable at the bedside, cheap, safe, accurate and add to decision making, said Dhaliwal. It should be automated and self-guided to different regions of the lung with clear sampling and external registration, with the ability to sense, image and sample, and part of routine care just like a tracheal suction catheter. The probes need to be cheap, specific, scalable and pharmaceutical grade. One application is neutrophil imaging and a probe has been developed that binds neutrophils. Neutrophil mediated lung injury is an important pathological process in numerous conditions and especially in ARDS.
Dhaliwal emphasised the importance of the multidisciplinary research team for this project, which includes together engineers, physical scientists and chemists.
Next steps
The team is currently testing the chemical probes with intensive care patients receiving mechanical ventilation, who are suspected of having pneumonia. A phase 2 trial is currently underway (Sensing Using Neutrophil Activation Probe on the Intensive Therapy Unit (SNAP-IT).
To sense, perturb and understand in vivo in situ process in human lungs is a long journey, but one we must take, concluded Dhaliwal.
Watch
Inflammation probe (SNAP-IT) and more videso from the Proteus YouTube panelLatest Articles
molecular imaging, ISICEM 2018, #ISICEM18, molecular alveoscopy, optical molecular biopsy
A medical imaging technology under development that enabled doctors to see bacteria deep inside patients' lungs for the first time was one of the highlights of ISICEM 2018.







