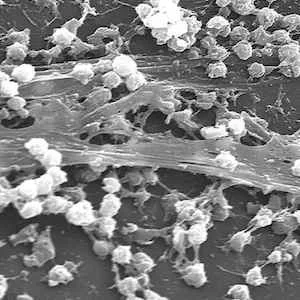
Dislodging Biofilms
Australian researchers have developed a technique that tricks bacterial biofilms, which form on living tissues and on medical devices and are linked to 80% of infections, into dislodging from their protective matrix, making them susceptible to treatment with antibiotics. The technique may have broad applications across a range of clinical and industrial settings.
The study was jointly led by Associate Professor Cyrille Boyer of the School of Chemical Engineering, University of New South Wales and deputy director of Australian Centre for NanoMedicine with Dr Nicolas Barraud, formerly of UNSW and now at France's Institut Pasteur, and is published in the open access journal Scientific Reports. Boyer explained that chronic biofilm-based infections are often extremely resistant to antibiotics and many other conventional antimicrobial agents, and have a high capacity to evade the body's immune system. He added: "Our study points to a pathway for the non-toxic dispersal of biofilms in infected tissue, while also greatly improving the effect of antibiotic therapies."
See Also: Banishing Bacterial Biofilms
When biofilms want to colonise a new site, they disperse into individual cells. This reduces the protective action of the biofilm. The researchers set out to trigger this process so that the bacteria would be vulnerable to antimicrobial agents. The researchers discovered how to dislodge biofilms by using the opportunistic human pathogen Pseudomonas aeruginosa. This is a model organism whose response to the technique the researchers believe will apply to most other bacteria.They injected into the biofilms iron oxide nanoparticles that were coated with polymers that help stabilise and maintain the nanoparticles in a dispersed and non-toxic state. Using an applied magnetic field they heated the nanoparticles and triggered the biofilms into dispersing.
S. Aureus Captured on the Move
For the first time, a study from the University of Nottingham has captured S. aureus moving. S. aureus, a spherical bacterium with no propulsive tail or appendages, up to now was considered to be a static organism. The researchers captured in a time-lapse video using high powered microscopy the progress of a ‘comet’ of Staph aureus cells moving across an agar surface over a period of 90 minutes from 8 hours post inoculation.This discovery could have implications for future clinical treatments, say the researchers, led by Dr. Steve Diggle from the University of Nottingham. The motility mechanism(s) of the bacteria could be a target for future vaccines and inhibitory pharmaceutical compounds.
If S.aureus has true motility as indicated by this research, says Dr. Diggle, it would be the first example of a Gram-positive bacteria with a typical Gram-positive cell wall moving without flagella or pili. Future research is needed into whether other Gram-positive organisms may be similarly motile.
Europe Lagging in Superbug Research Funding
National and European research funding in the field of antimicrobial resistance (AMR) is unbalanced and underfunded, according to a study from the Joint Programming Initiative on Antimicrobial Resistance, published in Lancet Infectious Diseases. The study mapped out antimicrobial resistance research undertaken across 19 countries from 2007-13, identifying 1,243 projects with a total public investment of €1.3 billion. The analysis showed that 66% of funding went to projects in the field of therapeutics. Transmission received 9% of the funding, 14% of funding went to diagnostics, 5% to interventions and only 2% was awarded to projects on antimicrobial resistance in the environment and 4% in surveillance.
“National research investment is too low compared to that committed at European Union level. To achieve greater impact, nations need to come together and pool available resources. This entails working together in a more efficient way to increase the impact of research through strengthening national and international coordination and collaborations as well as harmonising research activities and national strategies. The results demonstrate the need for a Joint Programming Initiative on Antimicrobial Resistance", said Herman Goossens, Chair of JPIAMR’s Science Advisor Board.
Sources: UNSW; University of Nottingham; Joint Programming Initiative on Antimicrobial Resistance.
Image credit: Biofilm on catheter, UNSW
Australian researchers have developed a technique that tricks bacterial biofilms, which form on living tissues and on medical devices and are linked to 80% of infections, into dislodging from their protective matrix, making them susceptible to treatment with antibiotics. The technique may have broad applications across a range of clinical and industrial settings.
The study was jointly led by Associate Professor Cyrille Boyer of the School of Chemical Engineering, University of New South Wales and deputy director of Australian Centre for NanoMedicine with Dr Nicolas Barraud, formerly of UNSW and now at France's Institut Pasteur, and is published in the open access journal Scientific Reports. Boyer explained that chronic biofilm-based infections are often extremely resistant to antibiotics and many other conventional antimicrobial agents, and have a high capacity to evade the body's immune system. He added: "Our study points to a pathway for the non-toxic dispersal of biofilms in infected tissue, while also greatly improving the effect of antibiotic therapies."
See Also: Banishing Bacterial Biofilms
When biofilms want to colonise a new site, they disperse into individual cells. This reduces the protective action of the biofilm. The researchers set out to trigger this process so that the bacteria would be vulnerable to antimicrobial agents. The researchers discovered how to dislodge biofilms by using the opportunistic human pathogen Pseudomonas aeruginosa. This is a model organism whose response to the technique the researchers believe will apply to most other bacteria.They injected into the biofilms iron oxide nanoparticles that were coated with polymers that help stabilise and maintain the nanoparticles in a dispersed and non-toxic state. Using an applied magnetic field they heated the nanoparticles and triggered the biofilms into dispersing.
S. Aureus Captured on the Move
For the first time, a study from the University of Nottingham has captured S. aureus moving. S. aureus, a spherical bacterium with no propulsive tail or appendages, up to now was considered to be a static organism. The researchers captured in a time-lapse video using high powered microscopy the progress of a ‘comet’ of Staph aureus cells moving across an agar surface over a period of 90 minutes from 8 hours post inoculation.This discovery could have implications for future clinical treatments, say the researchers, led by Dr. Steve Diggle from the University of Nottingham. The motility mechanism(s) of the bacteria could be a target for future vaccines and inhibitory pharmaceutical compounds.
If S.aureus has true motility as indicated by this research, says Dr. Diggle, it would be the first example of a Gram-positive bacteria with a typical Gram-positive cell wall moving without flagella or pili. Future research is needed into whether other Gram-positive organisms may be similarly motile.
Europe Lagging in Superbug Research Funding
National and European research funding in the field of antimicrobial resistance (AMR) is unbalanced and underfunded, according to a study from the Joint Programming Initiative on Antimicrobial Resistance, published in Lancet Infectious Diseases. The study mapped out antimicrobial resistance research undertaken across 19 countries from 2007-13, identifying 1,243 projects with a total public investment of €1.3 billion. The analysis showed that 66% of funding went to projects in the field of therapeutics. Transmission received 9% of the funding, 14% of funding went to diagnostics, 5% to interventions and only 2% was awarded to projects on antimicrobial resistance in the environment and 4% in surveillance.
“National research investment is too low compared to that committed at European Union level. To achieve greater impact, nations need to come together and pool available resources. This entails working together in a more efficient way to increase the impact of research through strengthening national and international coordination and collaborations as well as harmonising research activities and national strategies. The results demonstrate the need for a Joint Programming Initiative on Antimicrobial Resistance", said Herman Goossens, Chair of JPIAMR’s Science Advisor Board.
Sources: UNSW; University of Nottingham; Joint Programming Initiative on Antimicrobial Resistance.
Image credit: Biofilm on catheter, UNSW
References:
Nguyen TK, Duong HTT, Selvanayagam R, Boyer C, Barraud N (2015) Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Scientific Reports
5, Article number: 18385.
Pollitt EJG, Crusz SA, Diggle SP (2015) Staphylococcus aureus forms spreading dendrites that have characteristics of active motility. Scientific Reports 5, Article number: 17698.
Kelly R, Zoubiane G, Walsh D, Ward R, Goossens H (2015) Public funding for research on antibacterial resistance in the JPIAMR countries, the European Commission, and related European Union agencies: a systematic observational analysis. Lancet Infect Dis, Published online December 18. http://dx.doi.org/10.1016/
S1473-3099(15)00350-3
Pollitt EJG, Crusz SA, Diggle SP (2015) Staphylococcus aureus forms spreading dendrites that have characteristics of active motility. Scientific Reports 5, Article number: 17698.
Kelly R, Zoubiane G, Walsh D, Ward R, Goossens H (2015) Public funding for research on antibacterial resistance in the JPIAMR countries, the European Commission, and related European Union agencies: a systematic observational analysis. Lancet Infect Dis, Published online December 18. http://dx.doi.org/10.1016/
S1473-3099(15)00350-3
Latest Articles
Dislodging Biofilms Australian researchers have developed a technique that tricks bacterial biofilms, which form on living tissues and on medical devices...