ICU Management & Practice, Volume 22 - Issue 5, 2022
Ultrasonographic assessment of the neck vessels in critically ill patients contributes to rapid and non-invasive management of fluids to evaluate responsiveness and tolerance and blood volume status.
Introduction
There are multiple ways to evaluate the responsiveness and tolerance to IV fluids, as well as venous congestion, in critically ill patients. For these purposes, some static variables have traditionally been used, such as central venous pressure (CVP) and inferior vena cava diameter (IVCd), up to dynamic variables like pulse pressure variation (PPV), stroke volume variation (SVV), passive leg raising (PLR) combined with continuous measurement of cardiac output (CO) and stroke volume (SV), inferior vena cava variation index (IVCvi) and internal jugular vein variation index (IJVvi), velocity time integral variation (VTIv) of the left ventricle outflow tra ct (LVOT) and internal carotid artery peak systolic flow variation (ICASFv).
For a long time, placing a central venous catheter to measure CVP or placing a catheter in the pulmonary artery to measure pulmonary artery occlusion pressure were parameters to decide upon the administration of IV fluids in shock patients (Rivers et al. 2001); in present day, CVP is considered a reference point to stop IV resuscitation, in addition to evaluating venous congestion; later, the dynamic variable PPV was widely used to assess fluid responsiveness (Michard et al. 1999; Yang and Du 2014). Both techniques have the disadvantage of requiring an invasive device; furthermore, they imply additional financial costs. In the last decade, point-of-care ultrasound (POCUS) has been proposed as a non-invasive, low-cost device to evaluate fluid responsiveness and tolerance, such as the assessment of the inferior vena cava (IVC) and transthoracic echography (TTE), which require some degree of expertise; nonetheless, neck vessels are relatively easy to assess with ultrasound, and may provide prompt information for decision-making in critically ill patients, while properly correlating with other methods.
Ultrasonographic Assessment of Neck Vessels to Evaluate Fluid Responsiveness and Tolerance
Positive responsiveness to fluids is defined as an increase in SV of 10 to 15%, and therefore of CO after the administration of an IV fluid challenge, usually 5 to 10% of the estimated blood volume. The neck is an accessible anatomical region in most critically ill patients in Intensive Care Units (ICU), emergency departments (ED) and operating rooms (OR), unlike the ultrasonographic evaluation of the IVC and transthoracic echocardiography which may not be feasible or may be technically difficult to obtain images from, such as in patients with abdominal or chest pain, patients with abdomen or chest surgeries, patients on mechanical ventilation (MV) or on prone position, obese or ascites patients, or patients with chest wall deformities or with a poor anatomic window. Performing an ultrasonographic scan to the internal jugular vein (IJV) and the common carotid artery (CCA) could be much easier and faster than other methods and may provide valuable information to manage fluid therapy in critically ill patients, particularly in patients with circulatory shock (Table 1).
Evaluation of Fluid Responsiveness With the Internal Jugular Vein
IJV is a superficial vessel that lies beneath the sternocleidomastoid muscle, and it can be easily observed with an ultrasound. Cyclic changes in the pressure and volume of the intrathoracic systemic venous compartment induced by mechanical ventilation or spontaneous breathing can be transmitted to extrathoracic veins, which makes the evaluation of blood volume possible.
Technique
For this approach, a high-frequency linear transducer (10 MHz) with B and M modes is required. With the head of the patient at 30 to 45°, the transducer is placed transversely to the trachea at the level of the cricoid cartilage, to subsequently slide laterally to the line that goes along the angle of the mandible until observing the IJV and the CCA, the former being usually more lateral, of greater diameter, oval in shape, collapses on compression, distends with Valsalva manoeuvre, has a continuous flow and has thinner walls compared with the CCA. Once the image of the jugular vein is cantered, caution must be taken not to press it with the transducer to avoid unnecessary manipulation, in order to reduce the risk of a vasovagal reflex; for these purposes, we suggest leaning the operating hand in some bone structure or on the patient’s bed. We then switch to M-mode and measure the maximum and minimum IJV diameter.
Internal jugular vein diameter variation index (IJVdvi), which can be calculated with the formula: (maximum diameter - minimum diameter)/[(maximum diameter - + minimum diameter)/2)]×100, predicts fluid responsiveness capacity in postoperative patients and patients with controlled MV, with sensitivity of 91.43%, specificity of 82.86%, and AUC of 0.88 (CI 0.78–0.94) with a cut-off point of > 12.99 %, and shows good correlation with SVV (r = 0.51, p < 0.01 and AUC 0.88 vs. 0.97, p = 0.03) with good agreement between the variability of measurements made by two evaluators (Ma et al. 2018).
IJVdvi measurement can be performed with the formula: [(maximum diameter–minimum diameter)/maximum diameter)] ×100. Also known as IJV collapsibility index, it predicts adequate fluid responsiveness with cut-off points from >9.7% to >28.7% in patients under controlled MV, >11.4% in patients on spontaneous mode MV, and >36% in patients without MV (Haliloglu et al. 2017; Iizuka et al. 2020).
IJVdvi has also been described with the following formula:
[(maximum diameter–minimum diameter)/minimum diameter]×100
Also known as IJV distensibility index, it has a cut-off point of >18% for prediction of fluid responsiveness in patients with controlled MV and sepsis, with sensitivity of 80% and specificity of 95% for fluid responsiveness prediction (Guarracino et al. 2014).
Note: multiple authors describe internal jugular vein diameter variation as “collapsibility” or “distensibility” according to the phase of the respiratory cycle and MV mode, which may lead to confusion, thus we propose to only name it as internal jugular vein diameter variation index (IJVdvi), whilst taking into consideration the cut-off points referred to in the studies according to the type of patient.
Measurement of the Internal Jugular Vein Diameter Ratio for Assessment of Venous Congestion
It has been determined that a CVP >8mmHg is associated with greater risk of acute kidney injury, while CVP >10 mmHg has been associated with greater mortality risk. The previously proposed goals of 8-12 mmHg in early resuscitation in patients with sepsis are no longer recommended. One proposal to assess the systemic venous congestion is the IJV diameter ratio (IJVdr), which is the ratio between the maximum IJV diameter during Valsalva manoeuvre and the resting diameter at the end of the expiratory phase. It can be performed in patients without MV, with a low inter-rater error bias. Even in patients with heart failure, a good correlation with NT-proBNP plasma levels has been documented, with a cut-off point of <2 (Simon et al. 2010; Pellicori et al. 2014). Resting IJV diameter is low (0.10-0.15cm), and it usually increases up to 1 cm during Valsalva manoeuvre; this is similar between patients with and without heart failure. Normal IJVdr is >4, which identifies patients who have normal CVP and who may be able to tolerate fluids. When IJVdr is <4, it is considered abnormal, and if it reaches <2, it is considered a case of severe congestion. IJVdr calculation is performed with the following formula: IJV diameter during Valsalva/ IJV minimum diameter at the end of the expiration. Another alternative method to estimate CVP is the measurement of the internal jugular vein and common carotid artery diameter ratio, which is known as the IJV/CCA ratio, with a cut-off point of <1.75 to predict CVP <10 mmHg with sensitivity of 84.62% and specificity of 52.17%, positive predictive value (PPV) of 66% and negative predictive value (NPV) of 75% (Bano et al. 2018). Measurement of anteroposterior IJV diameter (AP-IJVd) measured at 2 cm above the clavicle, also correlates with CVP. An AP-IJVd <7 mm adequately correlates with a CVP <10 mmHg in patients without MV in the supine position (r= 0.92) (Donahue et al. 2008).
It is important to mention the limitations that the assessment of the IJV has, which are similar to that of the assessment of the IVC and the CVP. Any increase in the intrathoracic pressure can generate IJV distensibility and therefore underestimate its variation as occurs in mechanically ventilated patients with high positive end-expiratory pressure (PEEP), tension pneumothorax, severe air trapping, venous outflow obstruction such as venous stenosis, superior vena cava syndrome, cardiac tamponade, severe tricuspid regurgitation, heart failure, low lung compliance, arrhythmias, or jugular vein thrombosis. In the case of patients with spontaneous ventilation with vigorous respiratory effort, modifications in the IJV diameter will be significant and may overestimate its variation.
Evaluation of Fluid Responsiveness With the Internal Carotid Artery
CCA is another superficial, easy-to-access vessel for ultrasonographic assessment. It has been shown that in shock patients, deviation of blood flow is greater in this territory, which confidently reflects the status of systemic resistance and the respirophasic variation of SV.
Technique
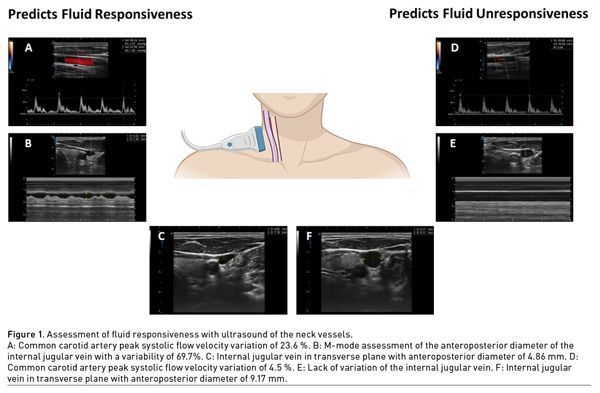
To perform this evaluation, a linear transducer and pulsed Doppler are required. With the head of the patient at 30°, the transducer is placed in the longitudinal plane of the internal carotid artery with the probe orientation marker towards the patient’s head, and pulsed Doppler is applied placing the sample volume at the centre of the vessel lumen with angle correction, observing the trace of the systolic flow spectrum and its variance with the patient’s respiratory cycle (Figure 1). Preferably, the sampling sweep is increased to more easily observe the peak systolic flow velocity (PSFV); the one with the highest velocity is identified and compared with the one with the lowest velocity by using the following formula:
CCApsfv = (Maximum psfv–Minimum psfv)/[( Maximum psfv+Minimum psfv)/2]×100
Common carotid artery peak systolic flow velocity variation (CCApsfv) with cut-off points of >11 to 14% predicts fluid responsiveness in patients under controlled MV with an excellent correlation with SVV (r = 0.84; p < 0.001). In spontaneous ventilation, cut-off points greater or equal to 13% for prediction of fluid responsiveness has been established (Song et al. 2014; Ibarra-Estrada et al. 2015).
Another assessment which is not affected by changes in spontaneous breathing is the measurement of the change in carotid flow time (CFTc), by measuring the maximum velocity; subsequently, passive leg raising is performed for 60 seconds. An increase in CFTc of more than 24.6% is expected in order to consider fluid responsiveness, with sensitivity of 60% and specificity of 92%.
On the other hand, in patients who are under MV, the end-inspiratory and end-expiratory occlusion manoeuvre can be performed, with a separation of 15 seconds, observing the pattern of change in pulsed Doppler flow velocity of the carotid artery with a cut-off point of 13% for precise fluid responsiveness prediction (Jalil et al. 2018).
The most important limitations of this technique, which can decrease its clinical predictive value, can be recalled with the mnemonics “LIMITS”- L: Low heart rate (HR)/respiratory rate (RR) (for instance, severe bradycardia, high ventilator frequency), I: Irregular heartbeats, M: Mechanical ventilation with low tidal volume or high total PEEP, I: Increased abdominal pressure, T: Thorax open, and S: Spontaneous breathing (Michard et al. 2015), which is why it is advisable to use at least two assessment techniques of fluid responsiveness to increase certainty in evaluation and clinical judgment (Michard et al. 2015).
Conclusion
Neck vessels ultrasonography is a simple, non-invasive technique that allows for evaluation of fluid responsiveness and tolerance in critically ill patients.
Conflict of Interest
None.
References:
Bano S, Qadeer A, Akhtar A et al. (2018) Measurement of Internal Jugular Vein and Common Carotid Artery Diameter Ratio by Ultrasound to Estimate Central Venous Pressure. Cureus. 10(3):e2277.
Bayraktar M, Kaçmaz M (2022) Correlation of internal jugular vein, common carotid artery, femoral artery and femoral vein diameters with central venous pressure. Medicine. 101(43):e31207.
Blehar DJ, Glazier S, Gaspari RJ (2014) Correlation of corrected flow time in the carotid artery with changes in intravascular volume status. Journal of Critical Care. 29(4):486-488.
Broilo F, Meregalli A, Friedman G (2015) Right internal jugular vein distensibility appears to be a surrogate marker for inferior vena cava vein distensibility for evaluating fluid responsiveness. Rev Bras Ter Intensiva. 27(3):205-211.
Donahue SP, Wood JP, Patel BM, Quinn JV (2009) Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am J Emerg Med. 27:851-855.
Guarracino F, Ferro B, Forfori F et al. (2014). Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 18(6):647.
Haliloglu M, Bilgili B, Kararmaz A, Cinel I (2017) The value of internal jugular vein collapsibility index in sepsis. Ulus Travma Acil Cerrahi Derg. 23:294-300.
Horejsek J, Kunstyr J, Michalek P, Porizka M (2022) Novel Methods for Predicting Fluid Responsiveness in Critically Ill Patients- A Narrative Review. Diagnostics (Basel). 12(2):513.
Ibarra-Estrada MÁ, López-Pulgarín JA, Mijangos-Méndez JC et al. (2015) Respiratory variation in carotid peak systolic velocity predicts volume responsiveness in mechanically ventilated patients with septic shock: a prospective cohort study. Crit Ultrasound J. 7(1):29.
Iizuka Y, Nomura T, Sanui M et al. (2020) Collapsibility of the Right Internal Jugular Vein Predicts Responsiveness to Fluid Administration in Patients Receiving Pressure Support Ventilation: A Prospective Cohort Study. J Clin Med Res. 12(3):150-156.
Jalil B, Thompson P, Cavallazzi R et al. (2018) Comparing Changes in Carotid Flow Time and Stroke Volume Induced by Passive Leg Raising. Am J Med Sci. 355(2):168-173.
Jozwiak M, Depret F, Teboul JL et al. (2017) Predicting Fluid Responsiveness in Critically Ill Patients by Using Combined End-Expiratory and End-Inspiratory Occlusions With Echocardiography. Crit Care Med. 45(11):e1131-e1138.
Kenny JS, Barjaktarevic I, Eibl AM et al. (2020) A wearable carotid Doppler tracks changes in the descending aorta and stroke volume induced by end-inspiratory and end-expiratory occlusion: A pilot study. Health Sci Rep. 3(4):e190.
Kent A, Patil P, Davila V et al. (2015) Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 10(1):44-49.
Ma GG, Hao GW, Yang XM et al. (2018) Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann Intensive Care. 8(1):6.
Michard F, Chemla D, Richard C et al. (1999) Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med. 159:935-9.
Michard F, Chemla D, Teboul JL (2015) Applicability of pulse pressure variation: how many shades of grey? Crit Care.19(1):144.
Pellicori P, Kallvikbacka-Bennett A, Zhang J et al. (2014) Revisiting a classical clinical sign: jugular venous ultrasound. Int J Cardiol. 170(3):364-370.
Simon MA, Kliner DE, Girod JP et al. (2010) Detection of elevated right atrial pressure using a simple bedside ultrasound measure. Am Heart J. 159(3):421-427.
Simon MA, Schnatz RG, Romeo JD, Pacella JJ (2018) Bedside ultrasound assessment of jugular venous compliance as a potential point-of-care method to predict acute decompensated heart failure 30-day readmission. J Am Heart Assoc. 7:e008184.
Song Y, Kwak YL, Song JW et al. (2014) Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. British Journal of Anaesthesia. 113(1):61-66
Thompson P, Cavallazzi R, Guardiola J et al. (2015) Diagnostic Value of Passive Leg Raise Induced Changes in Carotid Artery Flow Time to Predict Fluid Responsiveness in Critically Ill Patients, Chest. 148(4_MeetingAbstracts):231A.
Yang X, Du B (2014) Does pulse pressure variation predicts fluid responsiveness in critically ill patients: a critical review and meta-analysis. Crit Care. 18:650.












