ICU Management & Practice, Volume 23 - Issue 2, 2023
Kidney Replacement Therapy is a commonly used therapeutic strategy in the intensive care unit for patients who develop Acute Kidney Injury or who already have a diagnosis of chronic kidney disease. ICU staff should know when to use it and which type is most suitable for the circumstances.
Epidemiology and Outcomes of AKI
Acute kidney injury (AKI) is a common complication in critically ill patients. Up to 20-70% of patients will develop some stage of AKI in the intensive care unit (ICU) (Nisula et al. 2013; Libório et al. 2014; Kellum et al. 2015; Bouchard et al. 2015; Hoste et al. 2015). The requirement of kidney replacement therapy (KRT) in the ICU has been reported between 5-15% and will depend largely on the aetiology of the illness (Hoste et al. 2015). AKI has been associated with adverse clinical outcomes and mortality (Liangos et al. 2006). Mortality among critically ill patients and AKI is around 15-30% (Liaño and Pascual 1996; Uchino et al. 2005), rising up to 50-70% in patients that require KRT (Gaudry et al. 2016; Barbar et al. 2018; STARRT-AKI Investigators 2020; Cheng et al. 2020). The association between AKI and mortality in critically ill patients is likely due to multiple factors and not a direct causation; the severity of critical illness is one of the main factors involved in this association (Uchino et al. 2005; Parker et al. 1998).
Kidney Replacement Therapy in AKI
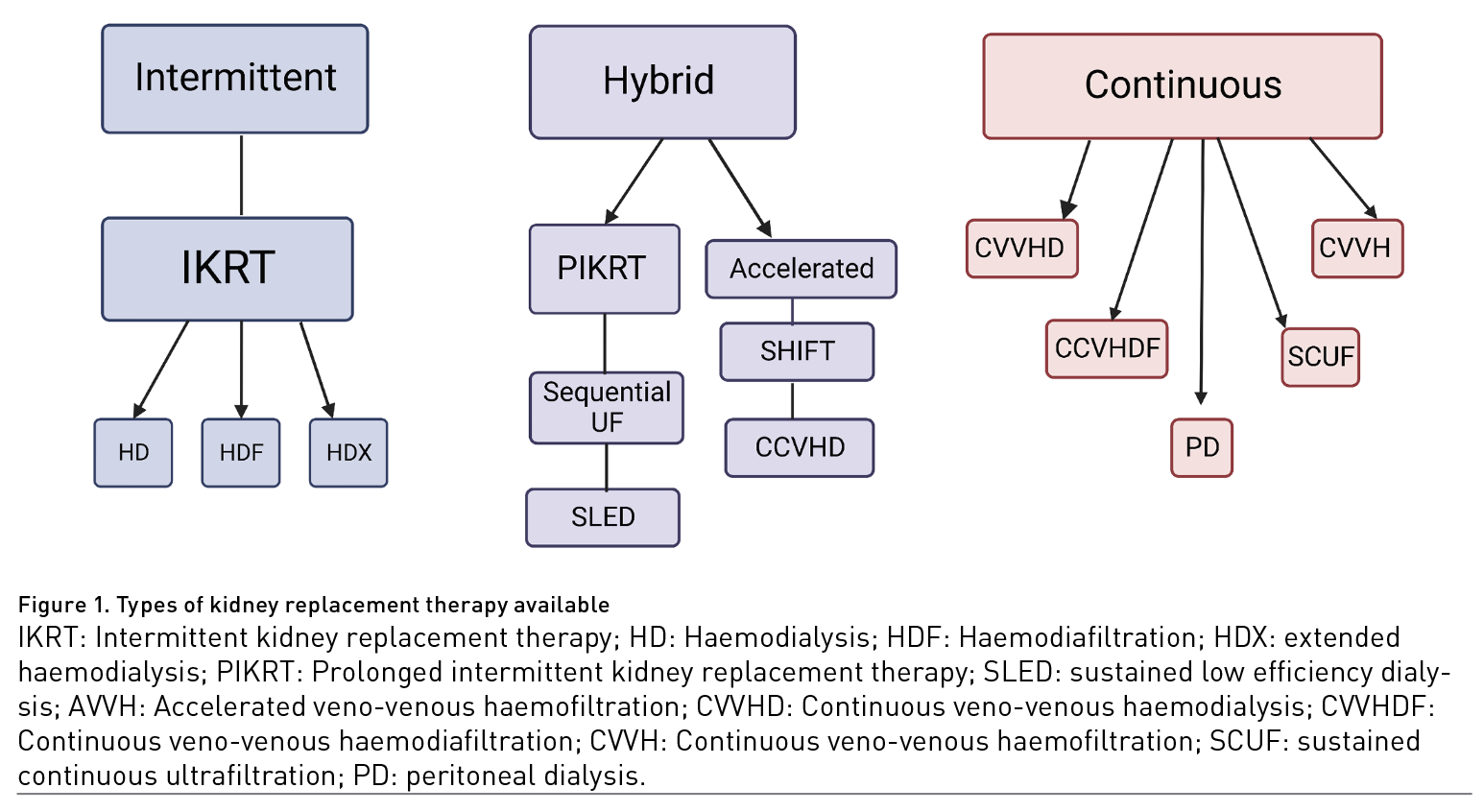
Types of KRT available can go from intermittent haemodialysis, continuous kidney replacement therapies (CKRT) (including peritoneal dialysis) and hybrid therapies that share characteristics of both intermittent and continuous methods (Figure 1). The type, modality, dose and timing of KRT have been widely explored as potential improvement variables in patients with AKI.
Indications of KRT
The indications for initiating KRT in the ICU are not perfectly defined. It is reasonable to consider therapy when a life-threatening circumstance arises, such as refractory hyperkalaemia and metabolic acidosis, despite medical treatment (e.g., diuretic therapy, IV sodium bicarbonate, etc.), blood urea nitrogen (BUN)>140 mg/dL with persistent oliguria, pulmonary oedema, and other complications of fluid overload (Gaudry et al. 2021). It is reasonable to initiate therapy in a critically ill patient with progressive AKI accompanied by oliguria or anuria and a positive fluid balance that is expected to continue to increase in the coming days. On the other hand, if the patient shows improvement in urinary flow, delaying the initiation of renal replacement therapy could be considered.
Type of Therapy
A systematic review and meta-analysis failed to show any difference between intermittent therapies and continuous therapies in mortality or kidney recovery and only showed a potential benefit in mean arterial pressure and use of pressors when using continuous therapies (Rabindranath et al. 2007). At least two other meta-analyses comparing hybrid and intermittent therapies vs continuous therapies also failed to show improvement in mortality or kidney recovery (Zhang et al. 2015; Nash et al. 2017). A recent systematic review and network meta-analysis that included all modalities, including peritoneal dialysis (PD), showed slightly better outcomes with PD but with very low certainty of evidence (Ye et al. 2021). A secondary analysis of the AKIK trial and IDEAL-ICU trials showed better survival with intermittent therapies in patients with SOFA score between 3-10 and no difference in mortality among patients with SOFA scores above 10 (Gaudry et al. 2022).
Modality
When using blood-based therapies, solutes can be cleared by convection, diffusion or adsorption. Convective therapies have the ability to remove medium size molecules more efficiently than diffusive therapies (Brunet et al. 1999). The potential benefit of removing medium size molecules in critically ill patients with AKI, especially in inflammatory states, has been explored. A systematic review and meta-analysis failed to show any difference in mortality when using haemofiltration (convection) vs haemodialysis (diffusion) (Friedrich et al. 2012).
Dose
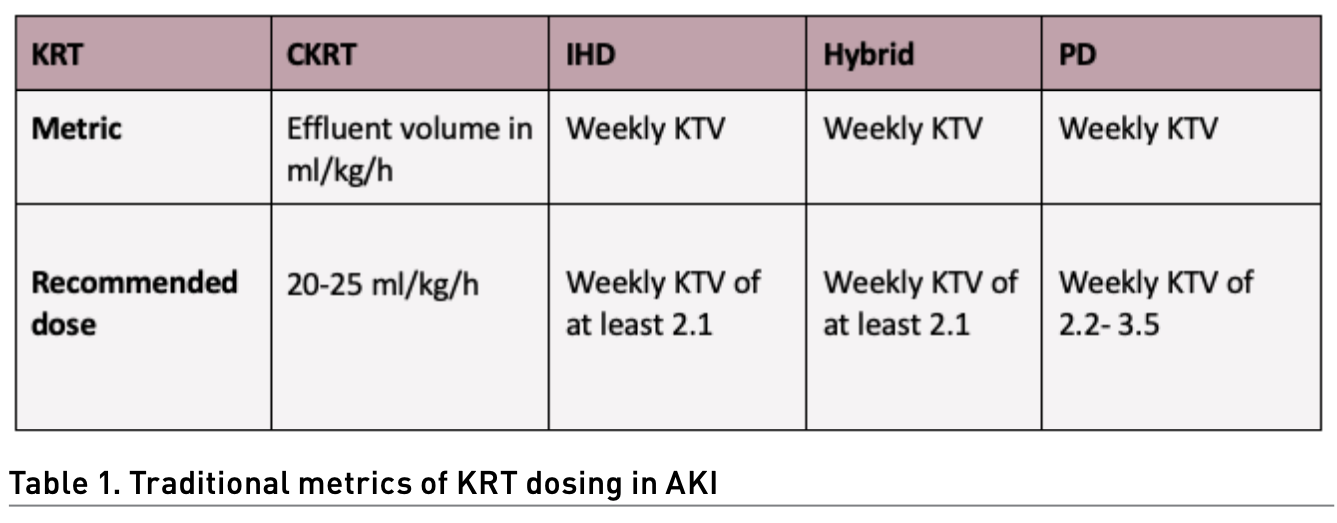
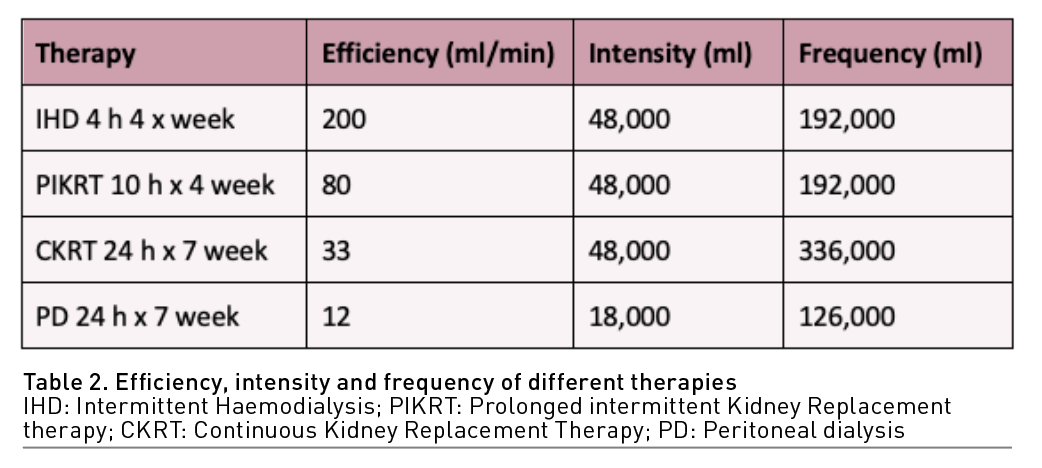
Dosing of KRT in AKI can be challenging, especially when using different types of KRT, mainly because traditional metrics of dosing can be different for every type of KRT (Table 1). Considering the nature of critically ill patients, higher doses have been proposed as an improvement clinical variable. In CKRT, giving more than 20-25 ml/kg/hr has failed to show any clinically relevant advantage in multiple studies and systematic reviews (Jun et al. 2010; Bellomo et al. 2009; Palevsky et al. 2009). In a clinical trial, intermittent haemodialysis (IHD) showed better outcomes when given daily (weekly KTV 5.8) versus alternate day (weekly KTV 3) but concluded that the results reflected the expected hazard associated with inadequate dosing of therapy rather than a benefit to an augmented dose of therapy (Schiffl et al. 2002). In hybrid therapies, a study failed to show any difference in survival or kidney improvement when comparing standard extended dialysis (daily treatment and target BUN < 56-70 mg/dl) vs intensified extended dialysis (two sessions per day and target BUN < 42 mg/dl) (Faulhaber-Walter et al. 2009). In PD, no difference in mortality was found when comparing intensified high-volume PD (weekly KTV 5.6) vs standard high-volume PD (weekly KTV 3.5) (Ponce et al. 2012); a later study showed that even minimal standard dosage (weekly KTV 2.2) was not inferior to standard high-volume PD (weekly KTV 3.5) (Parapiboon and Jamratpan 2017).
Timing
Early initiation of KRT (before traditional KRT indications) has been widely studied with overwhelming results proving no difference in survival or kidney recovery when compared to a late strategy, however systematically showing that nearly 50% of patients that were included in the late strategy never needed KRT (Gaudry et al. 2016; Barbar et al. 2018; STARRT-AKI Investigators 2020). AKIKI 2 trial showed no difference in survival between a late strategy (72 oliguric or BUN 112 mg/dl) and a very late strategy (BUN 140 mg/dl, overload, acidosis, hyperkalaemia) (Gaudry et al. 2021).
Rationale for Prescribing and Delivering KRT
To this day, we have learned that KRT will not give additional benefit to survival or kidney recovery no matter what type, modality, dose or timing is prescribed. Therefore indications, dosing and timing of KRT have to be focused only on solute and volume control (traditional indications). The type and modality of KRT will depend on technology and human resources available.
Technical and kinetic aspects of KRT
Solute and volume control can be achieved mainly by understanding and managing small molecule kinetics. The concepts of efficiency, intensity, frequency and efficacy are fundamental to understanding the different virtues and capacities of all the types of KRT (Pisitkun et al. 2004):
- Efficiency: is represented with clearance (K) (volume completely cleaned of a particular solute in a particular time) normally represented in ml/min. (K) will depend on variables related to the molecule itself (size, electric charge, molecular configuration), the host (volume of distribution, protein binding, half-life) and the clearance apparatus (blood and dialysate flow, type of membrane and mechanism of transport).
- Intensity: The total volume represented by the product of efficiency times the total time of therapy (K x total therapy time).
- Frequency: The total volume represented by the product of efficiency, intensity and the number of therapies given in a week (K x total therapy time x number of therapies in a week).
- Efficacy: represents the effective clinical outcome. Considering all the evidence to this day, the best efficacy metric in AKI and critically ill patients is volume and solute control.
Types of KRT need to be prescribed according to their capabilities to achieve efficacy. For example, to achieve solute and volume control, low-efficiency therapies such as CKRT and PD need high intensity to achieve the goal, while low-intensity therapies such as IHD need a high efficiency to achieve the same goal. Hybrid therapies will target both characteristics according to the particular clinical need (Table 2).
Kidney Replacement Therapy - Particular Aspects
Intermittent kidney replacement therapies
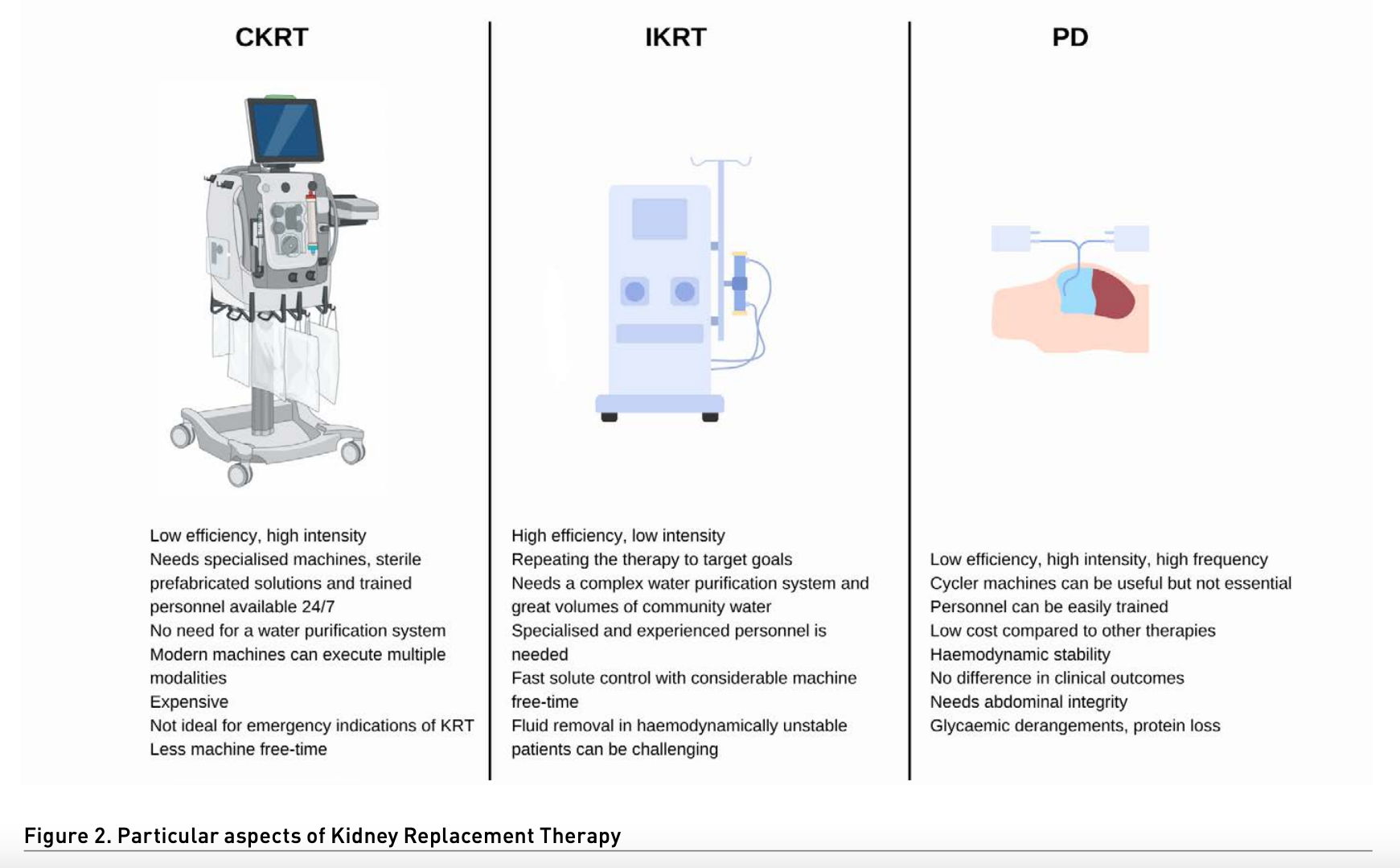
Mainly extrapolated from chronic haemodialysis, IKRT has been used in AKI since the beginning of dialysis. Modalities can include conventional haemodialysis, on line haemodiafiltration and extended haemodialysis.
- Kinetic characteristics: IKRT are high-efficiency and low-intensity therapies.
- Priorities when prescribing: optimising efficiency (blood flow, dialysate flow, vascular access, membranes) and repeating the therapy to target goals.
- Technical aspects: needs a complex water purification system and great volumes of community water; very specialised and experienced personnel are needed to deliver therapy.
- Pros: fast solute control with considerable machine free time.
- Cons: fluid removal in haemodynamically unstable patients can be challenging. Being a high-efficiency therapy, fast removal of solutes will considerably reduce the removal rate of solutes from other compartments (first-order kinetics), and most patients will require multiple sessions to maintain solute control.
Continuous kidney replacement therapy
From pump-less arteriovenous haemofiltration circuits to complex, highly technical machines, CKRT has been present in critically ill patients with AKI for quite some time now (Samoni et al. 2021). Modern machines opened the possibility for multiple modalities and options for prescription, including continuous veno-venous haemofiltration, haemodialysis, haemodiafiltration and sustained continuous ultrafiltration (SCUF).
- Kinetic characteristics: CKRT are low-efficiency and high-intensity therapies.
- Priorities when prescribing: being a therapy with very low efficiency, circuit patency is the main priority in these therapies (anticoagulation, filtration fraction, vascular access, monitoring, trained personnel).
- Technical aspects: CKRT needs specialised machines, sterile prefabricated solutions and trained personnel available 24/7.
- Pros: can achieve a very low ultrafiltration rate, osmolarity changes are subtle, no need for a water purification system, modern machines can execute multiple modalities.
- Cons: expensive therapy in comparison with other options, not ideal for emergency indications of KRT (acidosis, hyperkalaemia), considerably less free machine time.
Hybrid therapies
These therapies are born from the adaptation of available tools to achieve particular clinical needs. Hybrid therapies can go from IKRT machines trying to emulate CKRT and vice versa. Therefore, hybrid therapies can be grouped into prolonged intermittent therapies (PIKRT) and accelerated continuous therapies. Most common PIKRT protocols are sustained low efficiency dialysis (SLED), extended daily dialysis (EDD) and intermittent haemodialysis with sequential sustained ultrafiltration. Accelerated protocols include accelerated veno-venous haemofiltration and SHIFT CVVHD (Zhang et al. 2015; Gashti et al. 2008; Duran and Concepcion 2020).
- Kinetic characteristics: efficiency and intensity are variable and according to clinical needs.
- Priorities when prescribing: optimising efficiency and watching for circuit patency.
- Technical aspects: HD or CKRT ma-chines, off-label circuit adaptations, therapies less than 24 hours, trained personnel.
- Pros: best of both worlds with the tools available, without clinical implications, free machine time.
- Cons: centre protocol dependent, centre-to-centre variability, dosing medications can be challenging.
Peritoneal dialysis
PD has been used for AKI since 1946, but the introduction of extracorporeal therapies led to a drop in its use. Nonetheless, in low-income countries, acute PD never stopped being an option (Ponce et al. 2017). It was not until recent years with COVID-19, that developed countries turned to PD as a viable option. To this day, there is enough evidence of safety, viability and at least no inferiority when compared to other therapies (Ye et al. 2021; Gabriel et al. 2008; Ponce et al. 2013; George et al. 2011; Liu et al. 2017).
- Kinetic characteristics: very low-efficiency high intensity and high frequency.
- Priorities when prescribing: catheter patency, high volume and high-intensity therapy.
- Technical aspects: Requires experience in cath installation for surgical or percutaneous techniques, prefabricated sterile PD solutions, cycler machines can be useful but not essential, personnel can be easily trained, and therapy does not need continuous monitorisation.
- Pros: low cost compared to other therapies, haemodynamic stability, no difference in clinical outcomes with more complex and expensive therapies.
- Cons: needs abdominal integrity, can cause glycaemic derangements, protein loss, and rise in intra-abdominal pressure.
Conclusion
AKI in the ICU is very common, with very high mortality, especially when KRT is needed. Multiple efforts to improve outcomes in these patients by using KRT types, modalities, dosing and timing have failed. To this day, there is no evidence to support a particular type of KRT in patients with AKI. Therefore all efforts should be focused on solute and volume control with the technology, experience and personnel available. Each KRT type has particular kinetic and technical considerations that make them unique and should be prescribed, managed and monitored with a profound understanding of technical and clinical aspects.
Conflict of Interest
None.
References:
Angus DC, Linde-Zwirble WT, Lidicker J et al. (2001) Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 29(7):1303-1310.
Barnett K, Mercer SW, Norbury M et al. (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 380(9836):37-43.
Boumendil A, Latouche A, Guidet B (2011) On the benefit of intensive care for very old patients. Arch Intern Med. 171(12):1116-1117.
Brunker LB, Boncyk CS, Rengel KF, Hughes CG (2023) Elderly Patients and Management in Intensive Care Units (ICU): Clinical Challenges. Clin Interv Aging. 18:93-112.
Burki TK (2019) Post-traumatic stress in the intensive care unit. Lancet Respir Med. 7(10):843-844.
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 40(5):373-383.
Chen X, Mao G, Leng SX (2014) Frailty syndrome: An overview. Clin Interv Aging. 9:433-441.
Church S, Rogers E, Rockwood K, Theou O (2020) A scoping review of the Clinical Frailty Scale. BMC Geriatr. 20(1).
Ferrante LE, Pisani MA, Murphy TE et al. (2015) Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 175(4):523-529.
Flaatten H, de Lange DW, Artigas A et al. (2017a) The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 43(9):1319-1328.
Flaatten H, De Lange DW, Morandi A et al. (2017b) The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. 43(12):1820-1828.
Fronczek J, Polok K, de Lange DW et al. (2021) Relationship between the Clinical Frailty Scale and short-term mortality in patients ≥ 80 years old acutely admitted to the ICU: a prospective cohort study. Crit Care. 25(1).
Guidet B, van der Voort PHJ, Csomos A (2017) Intensive care in 2050: healthcare expenditure. Intensive Care Med. 43(8):1141-1143.
Guidet B, Vallet H, Boddaert J, et al. (2018) Caring for the critically ill patients over 80: a narrative review. Ann Intensive Care. 8(1).
Guidet B, de Lange DW, Boumendil A et al. (2020) The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 46(1):57-69.
Jorm AF (2004) The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): A review. Int Psychogeriatrics. 16(3):275-293.
Jung C, Guidet B, Flaatten H et al. (2023) Frailty in intensive care medicine must be measured, interpreted and taken into account! Intensive Care Med. 49(1):87-90.
Kotfis K, van Diem-Zaal I, Roberson SW et al. (2022) The future of intensive care: delirium should no longer be an issue. Crit Care. 26(1).
Krzych ŁJ, Rachfalska N, Putowski Z (2020) Delirium superimposed on dementia in perioperative period and intensive care. J Clin Med. 9(10):1-20.
Pandharipande PP, Girard TD, Ely EW (2014) Long-term cognitive impairment after critical illness. N Engl J Med. 370(2):185-186.
Pereira J V., Sanjanwala RM, Mohammed MK et al. (2020) Dexmedetomidine versus propofol sedation in reducing delirium among older adults in the ICU: A systematic review and meta-analysis. Eur J Anaesthesiol. 37(2):121-131.
Lambden S, Laterre PF, Levy MM, Francois B (2019) The SOFA score - Development, utility and challenges of accurate assessment in clinical trials. Crit Care. 23(1).
Le Guen J, Boumendil A, Guidet B et al. (2016) Are elderly patients’ opinions sought before admission to an intensive care unit? Results of the ICE-CUB study. Age Ageing. 45(2):303-309.
Lopes Ferreira F, Peres Bota D, Bross A et al. (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 286(14):1754-1758.
Marra A, Ely EW, Pandharipande PP, Patel MB (2017) The ABCDEF Bundle in Critical Care. Crit Care Clin. 33(2):225-243.
Mousai O, Tafoureau L, Yovell T et al. (2022) Clustering analysis of geriatric and acute characteristics in a cohort of very old patients on admission to ICU. Intensive Care Med. 48(12):1726-1735.
Muscedere J, Waters B, Varambally A et al. (2017) The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 43(8):1105-1122.
Nielsen AB, Thorsen-Meyer HC, Belling K et al. (2019) Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: a retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit Heal. 1(2):e78-e89.
Paul JA, Whittington RA, Baldwin MR (2020) Critical Illness and the Frailty Syndrome: Mechanisms and Potential Therapeutic Targets. Anesth Analg. 130(6):1545-1555.
Philippart F, Vesin A, Bruel C et al. (2013) The ETHICA study (part I): Elderly’s thoughts about intensive care unit admission for life-sustaining treatments. Intensive Care Med. 39(9):1565-1573.
Pisani MA, Inouye SK, McNicoll L, Redlich CA (2003) Screening for preexisting cognitive impairment in older intensive care unit patients: Use of proxy assessment. J Am Geriatr Soc. 51(5):689-693.
Rockwood K, Song X, MacKnight C et al. (2005) A global clinical measure of fitness and frailty in elderly people. C Can Med Assoc J. 173(5):489-495.
Salive ME (2013) Multimorbidity in older adults. Epidemiol Rev. 35(1):75-83.
Sanchez D, Brennan K, Al Sayfe M et al. (2020) Frailty, delirium and hospital mortality of older adults admitted to intensive care: the Delirium (Deli) in ICU study. Crit Care. 24(1).
Shepherd S, Batra A, Lerner DP (2017) Review of Critical Illness Myopathy and Neuropathy. The Neurohospitalist. 7(1):41-48.
Stavem K, Hoel H, Skjaker SA, Haagensen R (2017) Charlson comorbidity index derived from chart review or administrative data: Agreement and prediction of mortality in intensive care patients. Clin Epidemiol. 9:311-320.
Van Gassel RJJ, Baggerman MR, Van De Poll MCG (2020) Metabolic aspects of muscle wasting during critical illness. Curr Opin Clin Nutr Metab Care. 23(2):96-101.
Van Heerden PV, Beil M, Guidet B et al. (2021) A new multi-national network studying very old intensive care patients (vips). Anaesthesiol Intensive Ther. 53(4):290-295.
Vanhorebeek I, Latronico N, Van den Berghe G (2020) ICU-acquired weakness. Intensive Care Med. 46(4):637-653.
Vincent JL, Moreno R, Takala J et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 22(7):707-710.
Vincent JL, Creteur J (2022) Appropriate care for the elderly in the ICU. J Intern Med. 291(4):458-468.
Zador Z, Landry A, Cusimano MD, Geifman N (2019) Multimorbidity states associated with higher mortality rates in organ dysfunction and sepsis: A data-driven analysis in critical care. Crit Care. 23(1).
Zampieri FG, Colombari F (2014) The impact of performance status and comorbidities on the short-term prognosis of very elderly patients admitted to the ICU. BMC Anesthesiol. 14.